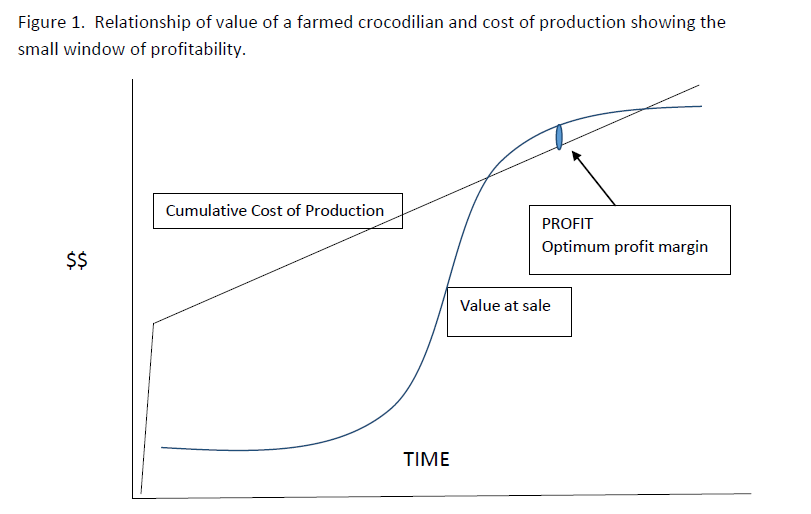
CCBM Section 66. ECONOMICS Introduction - James Perran Ross For the purposes of this introduction farming (closed cycle captive breeding) and ranching eggs collected from the wild are combined as the process of raising crocodilians from eggs or hatchlings to market size is essentially the same. More detailed treatment of farming and ranching are given in sections 4.4 and 6.1-6.5. The basic calculation for any commercial production is- PROFIT = SALE VALUE OF THE PRODUCT – COST OF PRODUCTION For agricultural/animal husbandry activities of all kinds, including raising crocodilians, this relationship must be considered in two calculations over a period of time from 'birth'/incubation to when an animal reaches market size.
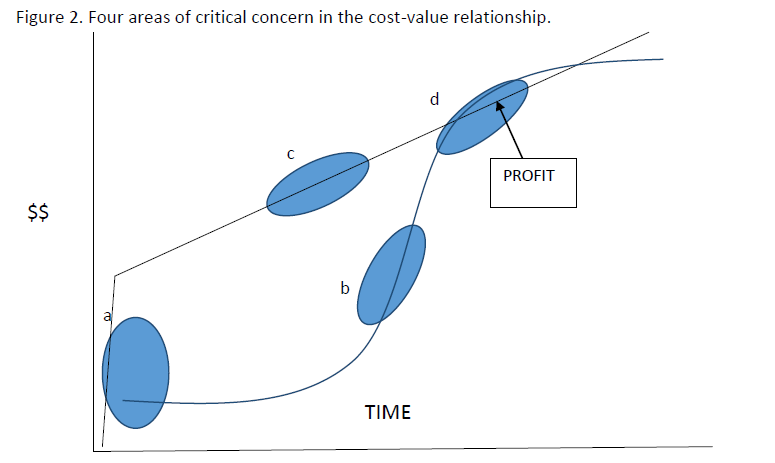
Initially, an egg or hatchling has a relatively low value and this increases slowly as the animal grows, but remains quite low, and then increases sharply as the animal approaches a size where it can be sold. The value then continues to increase slowly as the animal gets larger. The cost of production starts at a value well above zero because the costs of establishing a facility (purchasing land, building enclosures and infrastructure, bank loans to capitalize this development, labor, technical advice etc.) must be prorated among all the animals that the farm will produce. Every hatchling must pay a small portion of the up-front starting cost. From there, the cumulative costs of housing, feeding and maintaining the animal to market size increase in a roughly straight line manner. These two lines - Value and Cumulative Cost - determine at what point (if any) the value of each animal is greater than the cost of producing it and some profit can be made. However, as the animal continues to grow, the cumulative costs of producing increase while its value increases less quickly until the cost exceeds the value. Holding an animal in the farm beyond this point just costs money that will never be recovered and this situation is true for all animal husbandry- pigs, chickens, beef cows and crocodilians. This relationship is shown in Figure 1. And the area where value is greater than cost is obvious. We can consider in more detail, four areas of this graph that strongly influence the economic viability of a farming operation. Recognition, understanding and management of these factors is an important consideration for profitable operation: a.The effect of start-up costsb.The importance of high survival and high growth rates c. Maintenance expense during growth d. Fixed costs and economies of scale
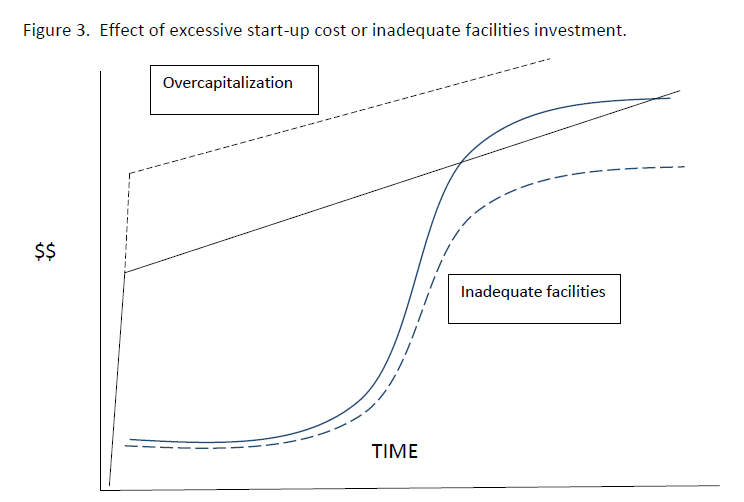
a. Start-up costs and investment in infrastructure. If the start-up costs of a facility per animal produced exceed the highest value at sale, then the farm can never make a profit. Overcapitalization and investment in extremely complex and expensive facilities is self-defeating. However, undercapitalization resulting in insufficient or inefficient operations can affect animal survival and growth rate, with profound effects on profit. An optimal start-up investment allowing adequate operation but not overwhelming value is location, crocodilian species and situation dependent. Careful research, development of business plans and recognition of uncertainty and instability in costs and sale prices are crucial to success. The other solution to this problem is to increase the number of animals produced so that the start-up cost is divided among more animals and therefore lower- but again overcrowding can negatively affect survival and growth. The effect of undercapitalization is equally dire. There are numerous examples of attempts to start village/artisanal level farms with very low levels of investment, facility and infrastructure and technical and husbandry knowledge. These invariably fail because the low survival and growth ensure the Value curve never intersects the Costs curve.
b. The
critical importance of high survival and high growth rates to economic
success. Many facilities can raise crocodiles but if mortality is high or growth rate low (and these often occur together as they have similar causes based in poor nutrition, crowding and generally inadequate husbandry) the economic impact is disastrous. Both the Costs and Value in our economic viability graph are per animal values- that is, they represent the dollar figures for sales value or expense divided among all the animals that can be brought to sale. If survival is low (or mortality high- same thing) the cost of raising all those dead animals up to the point when they died must be reflected in cost curve, but they contribute nothing to the value curve. Dead crocodilians represent lost revenue in the farm. The impact of slow growth is less direct but may be more detrimental. If growth rate is slow then Value curve shifts to the right and reduces the area of intersection with the cost curve and may even miss it altogether:
Note also that, at reduced growth rates, the time to reach
market size is longer and so the 'window of profitability' is shorter. A
farmer with higher growth rates has a wider window of opportunity to
seek favorable market prices. With slower growth the farmer has a
shorter period in which his stock is profitable and may have less
flexibility in negotiating sales prices. The optimal situation is to
sell stock when the difference between costs and value is greatest, but
practical considerations (eg cash flow, buyer pressure, different
market demand) may make sales at the edges of the profit window (ie
smaller or larger stock) economically sensible. What must be avoided is
pushing the slow growth rate out beyond the point where Value and Cost
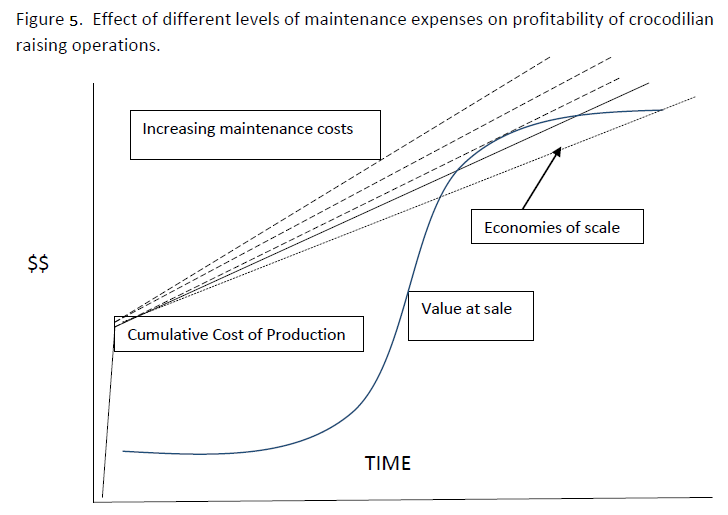
can intersect and many farmers have fallen into this trap. Maintaining high survival and growth is a complex highly technical and quite well understood process involving advanced knowledge and manipulation of diet, density of animals in pens, behavior (aggression), genetics (managing fast and slow growing hatchlings), disease management. One key question of debate is whether maintaining stock at a high temperature by artificial heating ensures a sufficiently high growth rate to offset this expense. For some species and situations (e.g. Alligator in Louisiana) this is clearly the case, but it may not work economically in all situations. There is huge wealth of research on these topics and abundant information available for specific countries and different species and types of facility. Successful farmers know and use this information to optimize growth and maintain high survival, with consequent economic benefits. Sections of this Capacity Manual address many of these issues and provide resources for information. c. Maintenance expenses during grow out. Growing crocodilians must be housed, provided with food, clean water, warmth and nutritional and medical supplements. This all costs money. Maintenance expenses can be considered to be fixed- i.e. independent of the number of animals held or variable- that is, changing as they are needed for a larger or smaller number of animals. Start-up costs are a fixed cost. Other fixed costs might be the cost of licenses, water supply, energy for lighting and heating and (within a range of number of crocodiles held) labor. Food is a variable cost - more animals need more food, and larger animals need more food. Other variable costs include tag and marking (eg PIT tags), waste water effluent treatment where this is required and veterinary/medical and nutritional supplement expense. One way to ensure the Cost and Value lines intersect to produce a profit is to minimize maintenance costs. Crocodile farmers are often preoccupied with costs of providing food. Certainly a reliable cost of high quality protein food is a necessary component of any farm. Many successful farms are associated with other animal production systems (eg chickens, pigs) so that the waste products of one operation (dead chickens, still births, abattoir waste etc.) can be the food source for the other (crocodiles) sometimes with an advantageous economy of waste disposal expenses. Like many other elements of crocodilian husbandry, finding the optimum balance among these costs and gains is a multidimensional and complex problem. Seeking information from researchers and other technical resources, sharing knowledge with effective farmers and carefully experimenting within a facility all contribute to successful solutions to this issue. d. Impact of fixed costs and economies of scale. The variable costs change with the number of animals in stock. With fewer crocodilians, lower costs and lower revenues should balance out, all other things being equal, and some farms successfully operate on low numbers and low margins by optimizing the other critical factors (start-up costs, maintenance, growth and survival). However, if the fixed costs can be distributed over a larger number of animals with no reduction in growth or survival, then the cumulative cost curve can be significantly lowered, reducing the time to reach the profit window and increasing the area beneath it. This can be accomplished with very careful attention to husbandry, and sometimes requires higher expenditures overall- but offset by lower per-animal costs. Operations in several countries, and most notably the extremely large operations captive breeding Caiman (Caiman crocodilus) in Colombia, demonstrate the effectiveness of this strategy and the details of how this was achieved are instructive. Key elements appear to be the availability of very abundant capital to build huge facilities, a large investment in technical knowledge (specifically of incubation and early nurturing methods) and an organism that was naturally adapted to high density.
The take-home message here is straightforward and universal for all animal husbandry operations. There are no simple formulas for success, each situation requires unique analysis and multi-dimensional or 'trade-off' solutions- but economically viable operations must plan and manage their start-up costs and investment, and optimize growth rate and survival to bring animals to market at the earliest opportunity when they have the greatest difference between cumulative costs and market value. This may not be the size at which the price per animal is highest- a consideration often overlooked by farmers. But what happens if animals are maintained beyond the first profitable size? With no other action, the cost of keeping them continues to grow as an unrecoverable loss. However, there are a number of ways to create a second intersection with the value curve. The daily cost of keeping large animals, prorated against all accumulated costs of all their siblings who were sold as skins at the optimum age/size is actually lower- that is, the cost curve, like the value curve, is actually Sigmoid ('S' shaped) when viewed over a long period. Additional 'values' can be created so that these larger animals become profit centers again. Two obvious added values for larger crocodilians are display value and as breeding stock for captive breeding. Facilities that have the capacity to display larger crocodilians and an audience willing to pay, can retrieve much of their accumulated costs. Similarly, an effective breeding adult can produce significant numbers of eggs for which the cost has already been paid over the life of the animal, and these, fed back into the production system, effectively reduce the start-up cost for a cohort of captive hatchlings, with the subsequent positive effect on the profit window. Advances in behavioral management, genetic testing and breeding, artificial insemination, and technical elements of incubation can all contribute to squeezing effective returns from larger animals. It is also instructive to examine the impact of regulatory activities on the economics of raising crocodilians. Because of their global status and international (CITES) and national protections, crocodilians require special regulatory regimes and these unavoidably impose expense on operators that they must calculate into business plans as part of cumulative costs. Costs of regulation include license and access fees, record keeping and inventory management expense, opportunity costs of inspection (lost time, disturbance to stock) and the special requirements for export permits and tags. Examination of our Cost-Value relationship also provides rational incentives for common, although usually illegal behavior. The obvious negative impact of high mortality can be offset by introducing new stock from the wild- either legally or illegally and the strong incentive to do so is evident. Ironically, and counter-intuitively, it can be argued that this driver also serves as a positive incentive for sustainable use and conservation of robust wild populations. If wild populations near a farm are accessible and numerous, the cost of 'replacements' is low but if the source of replacements is distant or depleted the cost may cause a detrimental up-step in the cumulative cost curve to the detriment of the profit window. Recognition and management of the interacting economic drivers,
careful and accurate accounting of real costs and knowledge based
manipulation of these factors can all contribute to economically viable
crocodilian farming and ranching. The following sections of this manual
provide additional detail on these factors. 6.1 Global markets, trade and economic factors – John Caldwell All crocodilians are listed in either Appendix I or Appendix II of the Convention on International Species of Wild Fauna and Flora (CITES) and many are found in international trade for the leather and fashion industry, for meat, and as live animals for breeding operations, food, the pet industry and zoos. The species involved in the skin trade are the 'classics': Alligator mississippiensis, Crocodylus acutus, C. moreletii, C. niloticus, C. novaeguineae, C. porosus and C. siamensis, and the caimans: Caiman crocodilus crocodilus, C. c. fuscus, C. latirostris, C. yacare and Melanosuchus niger. The trade in live animals involves relatively few individuals except for the Far Eastern trade in Siamese Crocodile C. siamensis. These animals are bred in captivity in Cambodia, Thailand and Viet Nam, and exported in large numbers to China where they are consumed as food. Crocodilian meat is traded widely but is particularly favoured in the
Far East, especially China and Hong Kong, S.A.R; the top species in
recent trade have been C. niloticus and C. siamensis. UNEP-WCMC produces
a report each year for the International Alligator and Crocodile Trade
Study (IACTS) funded by the Louisiana Department of Wildlife and
Fisheries. It examines the international trade in crocodilian skins,
with a particular focus on the most recent three-year period, but also
refers to trends over a longer timescale. It also attempts to identify
and highlight problem areas such as apparent discrepancies in reporting
and to recommend, where possible, workable solutions. The data used are
obtained from the searchable CITES Trade Database that is maintained by
UNEP-WCMC on behalf of the CITES Secretariat. Other information is
provided by the Crocodile Farmers Association of Zimbabwe and the United
Nations Food and Agriculture Organisation (FAO) Fisheries Statistical Division. It presents a detailed species-by-species analysis of the trade in skins and also of trade in other products such as live
animals and meat. The report is based on an analysis of the annual
reports submitted by the Parties to CITES. All direct, commercial trade
in whole skins and sides, live animals, meat and teeth of crocodilian
species is analysed, with two sides being considered to be equivalent to
one skin while trade in skins reported in other sub-units, such as
'tails', or in units of weight, area or length, are excluded. It
contains information on commercial trade from all sources, including
captive-bred, ranched and wild specimens. Wherever possible, data reported by the producer countries are used in preference to that reported by importing countries because there may be a time lag between when the export and the import are reported which may lead to the same skins being counted in different years and thus an overestimation of trade volume. However, where producer countries have failed to submit annual reports, or where exporter-reported trade volumes are substantially less than those reported by importers, importers' data are used. Many of the transactions are analysed at the export permit level, and, where possible, importer-reported data are corrected for year-end trade through permit analysis. Incomplete data due to late submission of, or failure to submit, CITES annual reports remains an impediment to conducting trade studies. Measures have been taken by the CITES Standing Committee to improve compliance with the reporting provisions of the Convention including reminders being sent by the Secretariat on behalf of the Standing Committee and a recommendation to suspend trade in CITES-listed species should a Party fail, without providing sufficient justification, to submit reports for three consecutive years (see CITES Resolution Conf. 11.17 (Rev. CoP16)) http://www.cites.org/eng/res/11/11-17R16.php. 6.2. Human/Community Livelihoods (see 4.2.1.above) (Alejandro Larriera) At one time, the conservation of wild crocodilians was pursued exclusively through the creation of wildlife refuges or sanctuaries, the imposition of strict bans on wild harvesting, and the belief that closed-cycle captive breeding was the only rational type of use. At that time commercial utilization of wild populations was regarded almost as the first step on the road to extinction. Subsequently, the concept of sustainability came into being, and in the case of many crocodilian species that were historically exploited, it became evident that rational utilization need not affect the status of the population. It was also evident that the real problem was environmental modification through deforestation, drainage of wetlands, or more recently, intensive agriculture. From that moment, "the enemy becomes a friend"; and commercial use was recognized as one of the very few effective tools against habitat loss. The challenge was to change people's attitudes towards crocodilians, and to give them more "value". Simply telling the public that crocodilians were "good"; for the environment was not enough. People needed more tangible rewards. They got these in several ways. The first step was not to deny simple facts: crocodiles are sometimes dangerous and can be a problem, so extensive educational awareness campaigns encouraged people to treat crocodiles with caution and respect. It is now evident that sustainable utilization of crocodilians through ranching or hunting, whether alone or combined with other activities, such as ecotourism, does work positively in favour of conservation. Despite the fact that, in general terms, there has always been significant objection to the idea of harvesting wildlife for conservation, and that there are many people who will argue that it doesn't work, actually it has shown to be highly successful in many instances. On the other hand, even those who still harbour hostility towards crocodilians, acknowledge their biological and economic importance, and would not wish to see them disappear. Such is the importance of linking conservation with people. (http://pubs.iied.org/14616IIED.html?k=CBNRM) Wild product trade involves a set of complex interlinkages between trade, local benefits, and conservation, meaning that knowing how to promote conservation and sustainable livelihoods through trade is often complicated. For example, increasing trade volumes can be positive for conservation where it increases incentives for local people to sustainably manage the resource, or it can be negative for conservation where it drives overexploitation and species decline. Which situation a particular trade context falls into will depend on what management regime and incentives are in place (among other factors). Likewise, regulatory interventions aimed at controlling harvest can be positive for local livelihoods, because they help ensure sustainability and control illegal take, or they can be negative for local benefits because they restrict options for generating income. Livelihood is one of the issues where CITES it was recently working on. (http://www.cites.org/eng/cop/16/doc/E-CoP16-19.pdf).
6.3. Ecotourism (Brian Jeffrey) Ecotourism, or nature-based tourism, is widely recognized as the most rapidly growing sector in the global tourism industry. Ecotourism has been embraced widely as a tool for commercial profit, community development, and environmental conservation (UNEP/WTO 2002). Many ecotourism products rely on protected areas, where environmental impacts are a concern. Pressure on conservation areas from ecotourism will continue to rise and an understanding of impacts and ways to manage them becomes increasingly important for land managers and tour operators (Eagles and McCool 2002; Buckley 2003; Buckley and King 2003). There is substantial literature on ecotourism as an element of the tourism industry and some key documentation are listed below (Fennell 1999; Weaver 2001a,b; Newsome et al. 2002). Drumm and Moore (2005) introduce the concept of ecotourism, present significant players, and give an overview of their roles in ecotourism planning and development. They deal with how and why ecotourism has evolved and what it means (Chapter 1), describe the various stakeholders involved in ecotourism management and development (Chapter 2), describe the roles of protected areas and their managers in ecotourism management and development (Chapter 3), provide an overview of the role that communities play in ecotourism management and development (Chapter 4), describe the role NGOs play in ecotourism management and development related to protected areas (Chapter 5), and provide a brief introduction to the tourism industry, its structure and its role in ecotourism development (Chapter 6). Drumm et al. (2004) introduce 6 principal management strategies, each of which is the backbone of an Ecotourism Management Plan (EMP). These strategies ensure that tourism activities contribute to conservation goals: Strategy 1 - overview of zoning for visitor use; Strategy 2 - explanation of visitor site planning and design to facilitate its management and limit impacts; Strategy 3 - the sustainable infrastructure design to decrease impact footprints; Strategy 4 - the revenue-generating mechanisms used to benefit the stakeholders; Strategy 5 - visitor impact monitoring and management; and, Strategy 6 - the importance of having naturalist guides. The authors also present 6 chapters with the goal to enable conservation professionals to participate in ecotourism planning process: 1. overview of business considerations in ecotourism; 2. roles conservation managers may have in business planning; 3. challenges involved with forming partnerships with tour operators; 4, 5 and 6 - focal elements needed in performing a feasibility study, creating a business plan, and financing an ecotourism business. IUCN and Kuoni (2012) is designed to help conservation groups, community based organizations, and protected area managers plan and run effective ecotourism businesses. Created as part of the Business Skills Transfer Program for Ecotourism Development (a joint effort by IUCN and Kuoni Group), it is intended to support conservation organizations in managing ecotourism related businesses through business skills, knowledge, and experience sharing. The book is divided into six sections: Understanding your Market and Product; Developing a Sustainable Business Plan; Running Sustainable Operations; Marketing and Sales; Human Resources; and, Health and Safety. These chapters are based on interviews with tourism industry professionals from various parts of the world. The sections include a discussion of why that particular topic is important, what it means, and how it applies to ecotourism businesses. Case studies, checklists, and a revision questions are found in each section. At the end of the book, there is a list of resources that may be useful in developing an ecotourism business. Gutirrez et al. (2005) was designed for field practitioners to perform a rapid assessment and analysis of tourism potential of a destination. The guidelines and tools provided are research based and reflect accepted criteria and principles of sustainability and ecotourism development. Sustainability concepts are incorporated to enhance tourism development to protect natural and cultural resources. The book is broken down into three phases. Phase I details assessment preparation. Phase II examines the assessment process that includes involving local stakeholders, attractions inventory, infrastructure and services, market demand analysis, supply and competitiveness, human and institutional capacity, socio-economic, cultural and natural resource use considerations, environmental and biodiversity footprint, and cost-benefit analysis. Phase III provides recommendations. Font et al. (2004). describes the 6 essentials for protected areas and tourism. Section 1 describes Tourism in Protected Areas. Section 2 details the Structures for Protected Area Management and Implications for Financing. Section 3 details specifics on Funding Methods. Section 4 summarizes previous sections and makes recommendations. Section 5 is a Case Study of Organisational Arrangements. Section 6 is a Case Study of Funding Methods. References Buckley, R.C. (2003). Ecological indicators of tourist impacts in parks. Journal of Ecotourism 2: 54-66. Buckley, R.C. and King, N. (2003). Visitor impact data in a management context. Pp. 89-101 in Nature-based Tourism, Environment and Land Management, ed. by R.C. Buckley, C.M. Pickering and D. Weaver. CAB International: Wallingford, UK. Drumm, A. and Moore, A. (2005). Ecotourism Development - A Manual for Conservation Planners and Managers. Volume 1: An Introduction to Ecotourism Planning. The Nature Conservancy: Arlington, Virginia, USA. Download. Drumm, A., Moore, A., Sales, A., Patterson, C. and Terborgh, J.E. (2004). Ecotourism Development - A Manual for Conservation Planners and Managers. Volume 2: The Business of Ecotourism Management and Development. The Nature Conservancy: Arlington, Virginia, USA. Download. Eagles, P.F.J. and McCool, S.F. (2002). Tourism in National Parks and Protected Areas: Planning and Management. CAB International, Wallingford, UK. Fennell, D.A. (1999). Ecotourism: an Introduction. Routeledge: New York. Font, X., Cochrane, J. and Tapper, R. (2004). Tourism for Protected Area Financing: Understanding Tourism Revenues for Effective Management Plans. Leeds Metropolitan University: Leeds. Download. Gutierrez, E., Lamoureux, K., Matus, E. and Sebunya, K. (2005). Linking Communities, Tourism and Conservation: A Tourism Assessment Process. Conservation International and The George Washington University: Washington D.C. Download. IUCN and Kuoni (2012). Integrating Business Skills into Ecotourism Operations. IUCN and Kuoni: Switzerland. Download. Newsome, D., Moore, S. and Dowling, R. (2002). Natural Areas Tourism: Ecology, Impacts and Management. Channel View: Clevedon UK. UNEP and WTO (2002). Quebec Declaration on Ecotourism. Download. Weaver, D. (2001a). Ecotourism. John Wiley and Sons: Brisbane. Weaver, D. (2001b). Encyclopedia of Ecotourism. CAB International: Wallingford, UK.
6.4. Ecological value of crocodilians (James Nifong) Crocodilians inhabit a wide range of aquatic ecosystems throughout the world from freshwater lakes, swamps, rivers, and wetlands to estuarine and fully marine ecosystems such as tidal rivers, mangroves, salt marshes, and coral reefs. Within all these ecosystems, as adults crocodilians occupy the most terminal position in associated food webs and are often referred to as top and/or apex predators. Crocodilians are carnivores and as such need to consume animal prey to maintain homeostasis, grow, and reproduce. Thus, all crocodilians interact with other animal species and in addition, many crocodilians modify their environment to better suit their needs. Through their interactions with prey and the environment, crocodilians provide many important ecological functions which are valuable to both humans and the environment. The ecological value of crocodilians can be categorized into four major components: effects on prey and food webs, habitat engineering and modification, cross-ecosystem linkages and nutrient dynamics, and ecological indicators. The following sections provide a brief overview of each of these components and links to resources which provide greater detail on each of these areas of interest. List of the major categories of crocodilian ecological roles/values:
6.4.1. Effects of crocodilian predator-prey interactions Summary: All living crocodilian species are carnivorous and most maintain diverse diets comprised of a variety of prey species ranging in size from small invertebrates (e.g., shrimp, beetles, snails) to large vertebrates (eg fish, mammals, turtles). While variation in diet is known to be driven by factors such as prey availability, habitat type, size, sex, and environmental conditions as well as differences between individuals, the fact remains crocodilians consume prey and interact with other species while doing so. As a result, the abundance and behavior of certain prey species can potentially be affected by the activities of crocodilians. In some cases these effects propagate through food webs, having the potential to influence community structure (ie abundance and diversity of plants and animals) and ecosystem functioning (i.e., the services ecosystems provide). While not thoroughly addressed in the literature, a number of studies have characterized specific examples of these types of interactions in various ecosystems. In-depth information: Effects of crocodilians on prey proceed through two pathways: 1) consumptive effects (ie capturing, killing, and consuming prey) and 2) non-consumptive effects (ie changes in prey behavior, growth, and/or morphology). For example, in Atlantic coast salt marshes of the United States the American alligator (Alligator mississippiensis) is a voracious predator of the Blue crab (Callinectes sapidus), with on average 48% of adult individuals found to feed on Blue crab. Using experiments Nifong and Silliman (2013) demonstrated that in addition to simply eating crabs, the presence of alligators also influenced crab behavior by reducing their activity and feeding rates. The combined effects of the consumptive and non-consumptive interactions between alligators and Blue crabs resulted in decreased mortality of crab prey species (ie snails and mussels); which in turn is hypothesized to contribute to variation in local community structure of salt marshes where alligators are present. This study is an example of the potential for indirect interactions (ie those interactions which arise through the use of a shared resource or connections with other consumers) stemming from crocodilians to impact entire food webs and ecological communities. As crocodilians are globally distributed and occupy many different ecosystems, future research will most certainly identify more examples of other crocodilian species affecting prey abundances and behavior. The pathways which give rise to crocodilian's effects on food webs are often not as straight forward as the previous example and result from many different indirect interactions with prey as well as non-prey species. For example, Bondavalli and Ulanowicz (1999) while studying A. mississippiensis in cypress swamps of Florida used network analysis to characterize the pathways contributing to the positive and negative effects of alligators on various prey species. Using this type of analysis the researchers discovered the potential for numerous cases of alligator enhancement of prey populations (44 in dry season and 64 in the wet season, to be exact), resulting in positive benefits for 19 prey taxa in the dry season and 22 in the wet season. Surprisingly, very few negative effects on prey were identified by the researchers in this system. Currently, we understand very little in regards to the potential implications of the indirect predator-prey interactions of crocodilians on food webs. Another pathway that the predator-prey interactions of crocodilian can influence food webs is through the transfer of nutrients and energy from areas of high to low productivity. Within all ecological systems, whether a lake, river, or swamp, photosynthetic primary producers (eg algae, plants, plankton) require energy (sunlight) and nutrients (ie elements and molecules) to grow and reproduce. Biomass produced by these organisms serves as the basal resource pools for all consumers within a particular food web and thus, primary production is a major determinant of the abundance and diversity of organisms a system can support. Crocodilians by feeding on prey in one system and transferring the nutrients and energy gained to a less productive system through excretion of metabolic waste products can provide important resource subsidies for primary producers within recipient food webs. For example, river-lakes within the Amazon region of South America are nutrient poor due to their hydrology and chiefly rely on organic materials (ie leaf litter, dead insects, etc.) washed into the lake from surrounding terrestrial habitats to support primary producers, mainly various taxa of phytoplankton, algae, and aquatic plants. Following dramatic declines of several caiman species (ie Caiman crocodilus, Paleosuchus palpebrosus, P. trigonatus, and Melanosuchus niger) and fish populations over the last century, Fittkau (1970) hypothesized that species of caiman by consuming terrestrial prey and migratory spawning fish are able to sequester externally produced biomass into the nutrient poor river-lake systems. Thus, the feeding habits of caiman can introduce much needed nutrients and energy into these systems, increasing primary production and enabling greater abundances of other consumers in the system (eg zooplankton, insects, crustaceans, fish). A number of other studies have also discussed the potential of crocodilians to serve as important vectors of nutrient and energy transfer between ecosystems (Subalusky et al. 2009; Rosenblatt and Heithaus 2011; Nifong et al. 2014). It is important to note that our understanding of the ecological implications of crocodilian predator-prey interactions is in its infancy and studies have only been performed on a handful of species within a few systems. Given the global distribution of crocodilian species and the diversity of ecosystems they reside in as well as diversity of prey they interact with, further study of these interactions will help to expand our understanding of the importance of crocodilians to ecological communities and the services those communities provide. References Bondavalli, C. and Ulanowicz, R. (1999). Unexpected effects of predators upon their prey: the case of the American alligator. Ecosystems 2: 49-63. Link: http://link.springer.com/article/10.1007/s100219900057 Fittkau, E.J. (1970). Role of caimans in the nutrient regime of mouth-lakes of Amazon affluents (an hypothesis). Biotropica 2: 138. Link: http://www.jstor.org/stable/2989771 Nifong, J. and Silliman, B. (2013). Impacts of a large-bodied, apex predator (Alligator mississippiensis Daudin 1801) on salt marsh food webs. Journal of Experimental Marine Biology and Ecology 440: 185-191. Link: http://www.sciencedirect.com/science/article/pii/S0022098113000063 Rosenblatt, A.E. and Heithaus, M.R. (2011). Does variation in movement tactics and trophic interactions among American alligators create habitat linkages? Journal of Animal Ecology 80: 786-798. Link: http://www.ncbi.nlm.nih.gov/pubmed/21418209 Subalusky, A.L., Fitzgerald, L.A. and Smith, L.L. (2009). Ontogenetic niche shifts in the American alligator establish functional connectivity between aquatic systems. Biological Conservation 142: 1507-1514. Link: http://linkinghub.elsevier.com/retrieve/pii/S0006320709000950 6.4.2. Crocodilians as ecosystem engineers Summary: Crocodilians are large predators capable of influencing their environment by consuming other species and causing them to change their behaviors. But crocodilians also frequently exert physical control over their environment, modifying their surroundings for their own benefit and sometimes the benefit of other species. These activities are collectively referred to as "ecosystem engineering."; Crocodilians physically change their environments in two main ways: 1) they dig holes, dens, and tunnels as refuges from environmental extremes and safe havens for offspring, and 2) they create nest sites either by digging holes in the ground (hole nests) or building mounds of soil and vegetation (mound nests). Crocodilian holes, dens, and tunnels can indirectly benefit other species that may attempt to also take refuge in them, especially if they contain water during periods of drought. Crocodilian nests are also frequently used by other species as their own nesting sites, allowing them to free-ride on the nest-building efforts of crocodilians. Furthermore, when crocodilians excavate holes, dens, and tunnels or pile soil and vegetation into mound nests they help to create topographic diversity across the landscape, allowing plant species with different habitat requirements to establish themselves. Thus, over time the ecosystem engineering activities of crocodilians can have profound effects on their environments and other animal and plant species, likely contributing to biodiversity and ecosystem diversity. In-depth information: Many crocodilians dig holes, dens, and tunnels as refuges from environmental extremes and safe havens from predators for themselves and offspring (reviewed by Platt 2000). American alligators (Alligator mississippiensis) are the most well-known hole diggers, especially alligators inhabiting the Everglades wetlands in southern Florida, USA. In the Everglades alligators typically dig and maintain holes in previously existing depressions in the substrate to create areas that will still contain water during the dry season. These "alligator holes"; allow alligators to thermoregulate more effectively, serve as refuge for other aquatic species that migrate to the holes from the surrounding wetlands as they dry, and act as feeding grounds for wading birds and mammals (Craighead 1968). Thus, in addition to providing a dependable supply of water the holes also supply alligators and other predators with a source of food (Craighead 1968). Alligator holes are typically 2-22 m diameter with depths up to 1.1 m and surface areas up to 1500 sq. m (Kushlan 1974; Campbell and Mazzotti 2004; Palmer and Mazzotti 2004). Thus, to dig and maintain a hole an individual alligator may excavate up to 300 m3 of material over a period of many years. The excavation process produces piles of vegetation and sediment around the edge of the alligator hole and these piles can be colonized by plants that require higher, drier ground to germinate (Craighead 1968; Campbell and Mazzotti 2004; Palmer and Mazzotti 2004). Many other crocodilians dig extensive and complex dens and tunnels, notably the Chinese alligator (Alligator sinensis) which can build tunnels 25-50 m long (reviewed by Platt 2000; Thorbjarnarson and Wang 2010). Hole nests and mound nests are created by every species of crocodilian and are the most ubiquitous impacts of crocodilians on the landscape. Mound nests range from small sizes measuring less than 1 m in diameter and 0.5 m in height (eg Thorbjarnarson et al. 2001) to impressively sized structures measuring 7 m long, 5 m wide, and 1 m tall (Kushlan and Mazzotti 1989). Thus, large nests can contain over 9 cubic metres of soil and vegetation collected from over 70 sq. m around the nest (Villamarin-Jurado and Suarez 2007). Hole nests are generally smaller with maximum hole openings 2.4 m in diameter (Ogden 1978) and depths of 1 m (Kushlan and Mazzotti 1989). Crocodilian nests are valuable to other species as they are regularly used as nesting sites by snakes, turtles, and lizards (eg Kushlan and Kushlan 1980; Brandt and Mazzotti 2000; Thorbjarnarson and Wang 2010). Also, after the eggs have hatched, abandoned mound nests are sometimes colonized by plants contributing to the formation of tree islands in wetland habitats (Craighead 1968). With up to 50% of females building nests in any given year (Lance 2003), crocodilian populations can therefore have extensive effects on the environments they inhabit and the population dynamics of other species that take advantage of crocodilian labour. Finally, it is important to note that crocodilians frequently consume fruits and seeds either intentionally or accidentally (Platt et al. 2013), suggesting that they may act as seed dispersers for certain plants, thereby contributing to the floral structure of their environments. Though recent experimental tests of crocodilian seed dispersal capabilities have not supported this possibility (Rosenblatt et al. 2014), much more research on this topic is needed. References Brandt, L. and Mazzotti, F.J. (2000). Nesting of alligators at the Arthur R. Marshall Loxahatchee National Wildlife Refuge. Florida Field Naturalist 28: 122-126. Campbell, M. and Mazzotti, F.J. (2004). Characterization of natural and artificial alligator holes. Southeastern Naturalist 3: 583-594. Link: http://www.jstor.org/stable/3878020 Craighead, F. (1968). The role of the alligator in shaping plant communities and maintaining wildlife in the southern Everglades. The Florida Naturalist 41: 3-7, 69-74. Kushlan, J. (1974). Observations on the role of the American alligator (Alligator mississippiensis) in the Southern Florida wetlands. Copeia 1974: 993-996. Link: http://www.jstor.org/stable/10.2307/1442609 Kushlan, J. and Kushlan, M.S. (1980). Everglades alligator nests: nesting sites for marsh reptiles. Copeia 1980:930-932. Link: http://www.jstor.org/stable/1444493 Kushlan, J. and Mazzotti, F.J. (1989). Population biology of the American crocodile. Journal of Herpetology 23: 7-2. Link: http://www.jstor.org/stable/1564310 Lance, V. (2003). Alligator physiology and life history: the importance of temperature. Experimental Gerontology 38: 801-805. Link: http://linkinghub.elsevier.com/retrieve/pii/S0531556503001128 Ogden, J. (1978). Status and nesting biology of the American crocodile, Crocodylus acutus, (Reptilia, Crocodilidae) in Florida. Journal of Herpetology 12: 183-196. Palmer, M. and Mazzotti, F.J. (2004). Structure of Everglades alligator holes. Wetlands 24: 115-122. Link: http://dx.doi.org/10.1672/0277-5212(2004)024[0115:SOEAH]2.0.CO;2 Platt, S. (2000). Dens and denning behavior of Morelet's crocodile (Crocodylus moreletii). Amphibia-Reptilia 21: 232-237. Platt, S., Elsey, R.M., Liu, H., Rainwater, T.R., Nifong, J.C., Rosenblatt, A.E., Heithaus, M.R. and Mazzotti, F.J. (2013). Frugivory and seed dispersal by crocodilians: an overlooked form of saurochory? Journal of Zoology 291: 87-99. Link: http://doi.wiley.com/10.1111/jzo.12052 Rosenblatt, A., Zona, S., Heithaus, M.R. and Mazzotti, F.J. (2014). Are seeds consumed by crocodilians viable? A test of the crocodilian saurochory hypothesis. Southeastern Naturalist 13: N26-N29. Link: http://www.bioone.org/doi/full/10.1656/058.013.0304%20 Thorbjarnarson, J., Wang, X. and He, L. (2001). Reproductive ecology of the Chinese alligator (Alligator sinensis) and implications for conservation. Journal of Herpetology 35: 553-558. Link: http://www.jstor.org/stable/1565892 Thorbjarnarson, J. and Wang, X. (2010). The Chinese Alligator: Ecology, Behavior, Conservation, and Culture. The Johns Hopkins University Press: Baltimore, MD. Villamarin-Jurado F. and Suarez, E. (2007). Nesting of the black caiman (Melanosuchus niger) in northeastern Ecuador. Journal of Herpetology 41: 164-167. Link: http://www.jstor.org/stable/4498567. 6.4.3. Crocodilian-mediated cross-ecosystem linkages Summary: Though they are cold-blooded sit-and-wait predators, crocodilians are capable of moving long distances to gain access to food and mates. In fact, recent research has shown that multiple crocodilian species regularly move between multiple ecosystem types, from freshwater to estuarine to marine areas, and therefore could act as biological vectors of connectivity across landscapes. Crocodilians could foster connectivity through three main pathways: 1) transporting nutrients between habitats by consuming prey in one habitat and excreting nutrients in another, 2) as adults, consuming prey and affecting the behaviors of prey species in different food webs, thereby linking the population dynamics of widely separated prey, and 3) shifting their habitat use as they grow such that prey consumed in the habitats preferred by juveniles subsidizes the consumption of prey consumed in the habitats preferred by adults, again linking the population dynamics of disparate prey populations. These potentially ecosystem-linking activities, when performed over long periods of time and by many crocodilians, could help stabilize and regulate ecosystem-level dynamics and processes. In-depth information: Many crocodilians travel long distances to access food and mating opportunities, sometimes repeatedly. These travels frequently cause crocodilians to traverse multiple ecosystem types, from freshwater to estuarine to marine environments. Therefore, crocodilians have the potential to connect different habitats through their movement and feeding patterns. For example, estuarine crocodiles (Crocodylus porosus) have been recorded regularly making trips of more than 50 km to get from freshwater areas of tidal rivers to the river mouth (Campbell et al. 2010). Similarly, American alligators (Alligator mississippiensis) on the coast of Florida, USA have been reported to "commute"; between freshwater and estuarine/marine environments, a roundtrip distance of approximately 30 km, up to 50 times in a 6-month period (Rosenblatt and Heithaus 2011; Rosenblatt et al. 2013). Through diet analysis researchers found that these alligators make these trips specifically to feed in estuarine/marine areas, and if the alligators return to freshwater areas relatively soon after feeding they may bring estuarine/marine derived nutrients with them (Rosenblatt and Heithaus 2011). Similar behaviors have been reported for alligators living on barrier islands in coastal Georgia, USA (Nifong et al. 2014). In addition to potentially transferring nutrients between habitats through these repeated movement patterns, crocodilians may be linking the population dynamics of disparate food webs if they actively feed in each habitat they traverse. Lastly, many crocodilians shift their habitat use as they mature from juveniles to adults, meaning that prey they consume in one type of habitat as juveniles subsidizes their ability to capture and consume prey in other types of habitats as adults. This type of ontogenetic niche shift establishes functional connectivity between disparate habitats and links the biogeochemical dynamics of multiple ecosystems (Subalusky et al. 2009). Citations and Links: Campbell H, ME Watts, S Sullivan, MA Read, S Choukroun, SR Irwin, and CE Franklin. (2010). Estuarine crocodiles ride surface currents to facilitate long-distance travel. Journal of Animal Ecology 79:955-964. Link: http://www.ncbi.nlm.nih.gov/pubmed/20546063 Nifong J, CA Layman, BR Silliman. Citation to follow. Rosenblatt, A., and M. R. Heithaus. (2011). Does variation in movement tactics and trophic interactions among American alligators create habitat linkages? Journal of Animal Ecology 80: 786-798. Link: http://www.ncbi.nlm.nih.gov/pubmed/21418209 Rosenblatt, A., M. R. Heithaus, F. J. Mazzotti, M. Cherkiss, and B. M. Jeffery. (2013). Intra-population variation in activity ranges, diel patterns, movement rates, and habitat use of American alligators in a subtropical estuary. Estuarine, Coastal and Shelf Science 135: 182-190. Link: http://linkinghub.elsevier.com/retrieve/pii/S0272771413004496 Subalusky, A., L.A. Fitzgerald, and L.L. Smith. (2009). Ontogenetic niche shifts in the American alligator establish functional connectivity between aquatic systems. Biological Conservation 142: 1507-1514. Link: http://linkinghub.elsevier.com/retrieve/pii/S0006320709000950
6.4.4 Crocodilians as indicators of ecosystem health Summary: Crocodilians are often considered the "canaries in a coal mine"; for aquatic ecosystems. This results from a number of biological and ecological attributes that enable crocodilians to function as important indicators of ecosystem health. First, unlike mammals or birds, crocodilians are ectothermic; meaning they rely on external sources of heat (ie sun) to sustain metabolic processes and regulate their body temperature through behavior, basking in the sun to warm and submerging in water or seeking shade to cool themselves. Consequently, the physiology and health of crocodilians is tightly linked to the environmental conditions they experience on a day to day basis. Moreover, sex determination (ie whether an embryo will be a female or male) in crocodilians is temperature dependent. Second, crocodilians are long-lived (naturally reaching ages of 30-70 years, http://crocodilian.com ) and many species attain large body sizes; thus, over a life-time an individual continually consumes prey and can accumulate substances present in prey tissues into their bodies. Third, crocodilians spend the majority of their life in or near aquatic habitats where they have the potential to directly interact with substances (eg pesticides, heavy metals, pollutants) present in the surrounding environment. Lastly, crocodilians require multiple types of habitat throughout their life-time due to changes in their ecological requirements as they grow. Specifically, as egg-laying vertebrates, female crocodilians must find suitable nesting sites close to nursery areas where newly hatched juveniles can seek refuge and avoid predation. As juveniles develop into sub-adults and adults, individuals must seek out new areas with adequate levels of prey and water resources to meet their growing needs. As a result the health and dynamics of crocodilian populations often relies on the environmental conditions of numerous ecosystems as well as the ability to freely move between them. A number of research programs use the assessment and monitoring of crocodilian health at the individual (ie body condition, growth rates, fertility, survivorship, contaminant load, etc.) as well as population level (i.e., population trends) to indicate human and ecosystem health concerns and to gauge the success of ecosystem restoration efforts. It should also be noted that crocodilians share many of the same or very similar physiological systems and signaling pathways as humans. In particular, hormone signaling by the endocrine system is highly conserved across vertebrates. As a result much of what we learn about how substances in the environment affect the physiology and health of crocodilians can be translated into our understanding of how humans may be affected by exposure to particular pollutants and contaminants in the environment. In-depth information: The monitoring of crocodilian health at the individual and population level is often used to provide a relative measure of ecosystem health and indicate human health concerns. A prime example where the monitoring of crocodilians health and population dynamics is used as an indicator of ecological health is from ongoing studies on populations of the American alligator (Alligator mississippiensis) and American crocodile (Crocodylus acutus) inhabiting the Everglades of Florida, USA. Specifically, the status and health of crocodiles and alligators inhabiting the Everglades National Park have been used in combination with other monitoring data to provide a relative measure of the success of efforts to restore historical patterns of freshwater flow. Wherein, during yearly surveys researchers count nests, measure hatchling survivorship and growth, and estimate body condition of individuals to develop general understanding of how restoration efforts are impacting the ecology of the Everglades system (Ogden 2005, Fujisaki et al. 2009, Mazzotti et al. 2009, Green et al. 2013). A number of other crocodilian species have been and are currently being used as indicator species. For example, in India population status of the gharial (Gavialis gangeticus) is used to designate areas of interest for conservation and as an indicator of ecosystem health (Sanghi 2014). Furthermore, the sudden disappearance and/or decline of crocodilians in a particular ecosystem can also be a strong indicator of habitat degradation and ecosystem change as with the case of Nile crocodiles (Crocodylus niloticus) in the Olifants River, South Africa (Ashton 2010). Crocodilians not only provide information on the health of ecosystems but can also be useful indicators of potential human health concerns such as high levels of pollutants or pesticides present in the environment. As long-lived organisms that continually grow throughout their lifetime, the tissues of crocodilians contain information on the environmental conditions present within a particular aquatic ecosystem. The reason why crocodilians are particularly useful is that as top predators substances often accumulate in body tissues. Known as bioaccumulation, this artifact of their biology and ecology can allow toxins to reach concentrations associated with negative health impacts and/or lethality even when concentrations in the environment are very small. For this reason crocodilians serve as a sort of natural record keepers and sentinels for ecosystem contaminants and pollutants. While not an exhaustive list, crocodilians have been used to detect and evaluate the concentrations of contaminants and pollutants such as heavy metals (Rainwater et al. 2002; Almli et al. 2005; Campbell et al. 2010), organochlorine pesticides (Pepper et al. 2004; Wu et al. 2006; Stoker et al. 2011), and chemicals such as DDT and polychlorinated biphenyls (PCBs) (Guillette et al. 1999). The continued monitoring of crocodilian populations serves to mitigating the potential negative human as well as ecosystem health impacts of contaminants and pollutants. Beyond the detection and monitoring of ecosystem contaminants, the study of crocodilians have provided essential information on the physiological impacts certain substances have on growth and development in vertebrate animals. For example, since the 1980s researchers have been studying the effects of endocrine-disrupting contaminants such as organochlorine pesticides and DDT on the reproductive biology of the American alligator (Alligator mississippiensis) by comparing the overall health, growth, and development of exposed and non-exposed populations (Guillette et al. 1985, 1995). Understanding the impacts of ecosystem contaminants of vertebrate health remains a highly active field of study and continued research using crocodilians as model species will most certainly yield important insights. As a final note, being ectothermic as well as having temperature dependent sex determination sets the stage for using crocodilians as a model to understand the potential impacts of climate change for ecosystems. For example, researchers in Argentina used data on the number of nests and clutch size of the Broad-snouted caiman (Caiman latirostris) to evaluate how patterns in precipitation and temperature influence reproductive success. Moreover, when combined with modeling techniques, researchers can use existing monitoring data on crocodilian populations to predict the effects of various climate change scenarios and evaluate the potential impacts on ecosystem structure and functioning (Richards et al. 2004). Citations and links: Almli, B., M. Mwase, T. Sivertsen, M. M. Musonda, and A. Flåøyen. 2005. Hepatic and renal concentrations of 10 trace elements in crocodiles (Crocodylus niloticus) in the Kafue and Luangwa rivers in Zambia. The Science of the Total Environment 337: 75–82. Link: http://www.ncbi.nlm.nih.gov/pubmed/15626380 Ashton, P.J. (2010). The demise of the Nile crocodile (Crocodylus niloticus) as a keystone species for aquatic ecosystem conservation in South Africa: The case of the Olifants River. Aquatic Conservation: Marine and Freshwater Ecosystems 20: 489-493. Link: http://doi.wiley.com/10.1002/aqc.1132 Campbell, J. W., M. N. Waters, A. Tarter, and J. Jackson. 2010. Heavy metal and selenium concentrations in liver tissue from wild American alligator (Alligator mississippiensis) livers near Charleston, South Carolina. Journal of Wildlife Diseases 46: 1234-1241. Link: http://www.ncbi.nlm.nih.gov/pubmed/20966273 Fujisaki, I., K. G. Rice, L. G. Pearlstine, and F. J. Mazzotti. 2009. Relationship between body condition of American alligators and water depth in the Everglades, Florida. Hydrobiologia 635: 329-338. Link: http://www.springerlink.com/index/10.1007/s10750-009-9925-3 Green, T. W., D. H. Slone, E. D. Swain, M. S. Cherkiss, M. Lohmann, F. J. Mazzotti, and K. G. Rice. 2013. Evaluating Effects of Everglades Restoration on American Crocodile Populations in South Florida Using a Spatially-Explicit, Stage-Based Population Model. Wetlands 34: 213-224. Link: http://link.springer.com/10.1007/s13157-012-0370-0 Guillette, L. J., J. W. Brock, A. A. Rooney, and A. R. Woodward. 1999. Serum Concentrations of Various Environmental Contaminants and Their Relationship to Sex Steroid Concentrations and Phallus Size in Juvenile American Alligators. Archives of Environmental Contamination and Toxicology 36: 447-455. Link: http://link.springer.com/article/10.1007/PL00006617 Guillette, L. J., D. A. Crain, A. A. Rooney, and D. B. Pickford. 1995. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environmental health perspectives 103: 157-164. Link: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1518862&tool=pmcentrez&rendertype=abstract Guillette, L. J., T. S. Gross, G. R. Masson, J. M. Matter, H. F. Percival, and A. R. Woodwardff. 1994. Developemental Abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environmental Health Perspectives 102: 680-688. Link: http://www.jstor.org/stable/3432198 Mazzotti, F. J., G. R. Best, L. A. Brandt, M. S. Cherkiss, B. M. Jeffery, and K. G. Rice. 2009. Alligators and crocodiles as indicators for restoration of Everglades ecosystems. Ecological Indicators 9: S137-S149. Link: http://linkinghub.elsevier.com/retrieve/pii/S1470160X08000770 Nifong, J. and B. Silliman. 2013. Impacts of a large-bodied, apex predator (Alligator mississippiensis Daudin 1801) on salt marsh food webs. Journal of Experimental Marine Biology and Ecology 440: 185-191. Link: http://www.sciencedirect.com/science/article/pii/S0022098113000063 Ogden, J. C. 2005. Everglades Ridge and Slough Conceptual Ecological Model. Wetlands 25: 810-820. Link: http://www.bioone.org/doi/abs/10.1672/0277-5212(2005)025%5B0810:ERASCE%5D2.0.CO;2 Pepper, C.B., T.R. Rainwater, S.G. Platt, J. a Dever, T. a Anderson, and S.T. McMurry. 2004. Organochlorine pesticides in chorioallantoic membranes of Morelet's crocodile eggs from belize. Journal of wildlife diseases 40: 493-500. Link: http://www.ncbi.nlm.nih.gov/pubmed/15465717 Rainwater, T. R., B. M. Adair, S. G. Platt, T. a Anderson, G. P. Cobb, and S. T. McMurry. 2002. Mercury in Morelet's crocodile eggs from northern Belize. Archives of environmental contamination and toxicology 42: 319-324. Link: http://www.ncbi.nlm.nih.gov/pubmed/11910460 Richards, P. M., W. M. Mooij, and D. L. DeAngelis. 2004. Evaluating the effect of salinity on a simulated American crocodile (Crocodylus acutus) population with applications to conservation and Everglades restoration 180: 371-394. Link: http://linkinghub.elsevier.com/retrieve/pii/S0304380004002881 Sanghi, R. 2014. Our National River Ganga. Page 417 (R. Sanghi, Ed.). Springer International Publishing, Cham. Link: http://link.springer.com/10.1007/978-3-319-00530-0 Stoker, C., M. R. Repetti, S. R. García, M. A. Zayas, G. H. Galoppo, H. R. Beldoménico, E. H. Luque, and M. Muñoz-de-Toro. 2011. Organochlorine compound residues in the eggs of broad-snouted caimans (Caiman latirostris) and correlation with measures of reproductive performance. Chemosphere 84:311-317. Link: http://www.ncbi.nlm.nih.gov/pubmed/21531435 Wu, T. H., J. E. Cañas, T. R. Rainwater, S. G. Platt, S. T. McMurry, and T. a Anderson. 2006. Organochlorine contaminants in complete clutches of Morelet's crocodile (Crocodylus moreletii) eggs from Belize. Environmental pollution (Barking, Essex?: 1987) 144: 151-157. Link: http://www.ncbi.nlm.nih.gov/pubmed/16504356
The following section is optional and incomplete at this time. Below is a sort of bare outline of some potential points of discussion/elaboration that could be expanded upon if the section is completed. Other suggestions are welcome. 6.4.5 Future directions and need for research -Crocodilians as umbrella species for conservation of threatened habitat and resources. -Crocodilians as model species to understand ecology of top predators and vertebrate physiology. -Research on links between croc ecosystem engineering activities and ecosystem health/resilience as well as fitness of prey species -Research on effects of climate change on croc behaviors/ecological roles. How will they fare in a warming, high sea-level world? -Research on the generality of crocodilian behavior and ecological interactions. Comparative studies across taxa and different ecosystems. 6.5 Commercial products overview (Don Ashley) Commercial trade in crocodilian products has existed for centuries, particularly in the mid-19th century when the durable leather was locally used for boots and other leathergoods. But the international trade in skins and leather only gained momentum after World War II, peaking by the early 1960s at 500,000 classic and 1.5 million caiman skins per year (Grawitz, Gordon Choisy, per. comm.). Unregulated, and later the same decade, illegal trade threatened many crocodilian species worldwide. A combination of state, national and international regulatory efforts significantly reduced the commercial trade in crocodilian skins, culminating in 1977 with most species (ie 15, including the American alligator and without any population exemptions) listed in Appendix I of the CITES Treaty ratified in 1975 to control trade that was detrimental to the survival of a species. Based primarily on a decade of alligator research at Rockefeller Refuge, Louisiana, by Ted Joanen and colleagues, and a management plan that proposed to use the economic incentive of legal trade to enhance the conservation of wetlands and benefit local people who depended on the marsh for their livelihoods, the USA successfully petitioned CITES in 1979 to downlist the American alligator to Appendix II (regulated trade). Subsequently, Australia, Papua New Guinea and several southern African countries incrementally proposed similar CITES downlistings through a combination of captive-breeding, ranching and limited wild harvest programs. Four species from those original range country downlistings (Alligator mississippiensis, Crocodylus porosus, C. novaeguineae and C. niloticus) today account for most world trade in classic crocodilian skins (Caldwell 2012). More recently C. siamensis (Southeast Asia), C. moreletii (Mexico) and C. acutus (South America) have entered the trade. Total world trade of all classics has averaged more than half a million skins per year in 2001-2010 (Caldwell 2012). By 2013 the estimated raw value of classic skins in international trade was more than $100 million per year, more than $200 million as leather and more than $1 billion as retail products, mostly marketed through the luxury brand sector. The caiman trade followed a similar path, with Venezuela originally instituting a management plan for Caiman crocodilus, and Colombia (C. c. fuscus), Argentina (C. latirostris, C. yacare), Bolivia (C. yacare) and others following. Total world trade in caiman has ranged up to 1 million skins per year, which lags a little behind the historical trade ratio of 3 to 1 caiman to classics (Y. Takehara, pers. comm.). From 2001 to 2010 the total average international caiman trade was about three quarters of a million skins a year (Caldwell 2012) with an estimated raw value of about $50 million per year. Global trade in crocodilian meat peaked in 2006 and 2007 at 1000 tons (Caldwell 2010) and averaged at least 500 tons a year since 2002. Domestic markets for crocodilian meat consume most production, particularly in the USA and Australia, so those quantities are not documented in CITES annual reports which are the basis of the WCMC/IACTS global trade reports. Meat yields and market values vary widely between species, range and importing countries so an estimate of total meat value is a guess at best. Alligator meat is worth more than $5 million per year at the processing level with wholesale and retail markups in the $10-15 million per year range. The Saltwater Crocodile (C. porosus) is the most valuable crocodile in trade by unit owing to its uniform, small scale pattern desired by the handbag trade. The Nile Crocodile (C. niloticus) is also favoured for handbags and accessories due to symmetrical belly patterns and volume (about 150,000 skins/year, 2001-2010). The American alligator (A. mississippiensis) is most valuable by volume and leather yields (about 300,000 skins/year, 2001-2010) mostly for watchbands and small leathergoods from smaller sizes (<30 cm belly width) as well as handbags, belts, footwear and accessories from larger sizes (greater than 30 cm belly width). Caiman products include all leathergoods sectors but are generally less value than classics due to the prevalence of buttons (osteoderms) in belly patterns. Only half of the world's crocodilian species are in world trade and originate from a supply that is not detrimental to the survival of a species. The greatest threat to crocodilian conservation is loss, degradation or fragmentation of natural habitats. Ranching of wild eggs and hatchlings or limited wild harvests provide direct links between the economic incentives of sustainable use to conservation of wetlands and benefits to local people. The crocodilian trade has generally been put forward as the best model for sustainable use that benefits both conservation and communities. Certainly most illegal trade has been stopped or diverted to trade that is legal, sustainable and verifiable (traceable and independently monitored). This was accomplished through a combination of exporting and importing country initiatives within the CITES framework for universal tagging, permits for export, re-export and sometimes import, as well as annual reporting and trade monitoring. The role of the IUCN-SSC Crocodile Specialist Group (CSG) has been central to this success by providing a forum for dialogue and cooperation between scientists, conservationists and the industry. This is unique among the wildlife trades with significantly underestimated value to ensuring world trade that is legal, sustainable and verifiable with benefits to conservation (natural habitats) and communities (local people). It is the "Marsh to Market" story of most crocodilians in trade that offers the best opportunity to better inform the public and consumers about the benefits of sustainable use as a counterpoint to trade bans and public attitudes about wildlife in trade. This remains the most significant challenge of the 21st century. References Caldwell, J. (2012). International Alligator Crocodile Trade. UNEP-World Conservation Monitoring Center: Cambridge. Caldwell, J. (2010). Global Trade in Crocodilian Meat. UNEP-World Conservation Monitoring Center: Cambridge.
6.5.1. Skins, quality, skinning, preparation, storage- unassigned (?) 6.5.2. Meat- (Cameron Carter) Ashley Pearcy 6.5.3 Trophies, curios and medicinals (Ashley Pearcy) Innovation from East to WestMedicinal uses of crocodiles and alligators have been recognized from ancient times, dating back to 1578. As recorded in the “Compendium of Materia Medica" or Ben Cao Gang Mu, a memory of the world register with UNESCO written by Li Shizhen, crocodile meat is good for the human body (Li, 1578). Other parts, including fat, blood, and organs have been used as a cure for a variety of ailments. Crocodile fat has played a prominent role in traditional medicine. The acknowledgement of fat composition having a noticeable effect on development in the crocodile itself (Ferguson et al. 1997) supports further studies into the benefits for humans. The fat and oils derived from the fat are considered a primary source for relief of respiratory and joint ailments for rural communities throughout crocodilian habitats worldwide (Ross 1992). To explore the validity of these claims, Buthelezi et al. (2012) tested the antimicrobial benefits of major fatty acids, orally and topically administered crocodile oil for inflammation, and found some justification for the continued use by traditional healers for these conditions, although research into the mechanics of crocodile fat effectiveness is still necessary. Crocodile blood has been
thoroughly studied in the past decade to identify its microbial properties.
Britton et al. (2002) identified high antibiotic activity in the blood of saltwater
crocodiles after noticing that crocodiles are not prone to infection. BBC news
reported their discovery of Crocodillin, a peptide which can kill bacteria without
damage to a normal cell (BBC News, 2000). Biochemists have reported the discovery of
the anti-microbial fractions in the blood of alligators and crocodiles (Merchant et al. 2003; Preecharam et al. 2010; Pata et al. 2011).
Merchant et al. also reported antiviral
activity of serum from the American alligator (Alligator
mississippiensis) (Merchant
et al. 2005).
Crocosin and Leucrosin are examples of fractions derived from blood of C. siamensis
which can kill bacteria (Preecharam et al. 2010; Pata et al. 2011).
Other than these, biochemical activities - eg anti-oxidation, anti-inflammation
properties of blood have been studied and the possibility of a new drug or
functional food is being sought. Crocodile meat, dried blood and other parts of Crocodylus siamensis have incremental demands for health concerning among Asian consumers. Biologist and food scientists (Temsiripong, Y. and T.) proposed an idea to improve the quality and safety for oral consumption of crocodile products and the possibility for commercialization by following the latest product development process. Freeze-dried crocodile blood in capsules was developed after confirmation of its efficacy e.g. anti-microbial peptides and heme iron supplement through collaboration with experts. The first FDA approved, freeze-dried crocodile blood in capsule, Modaplas®, was permitted for commercialization as a food supplement in 2005 by FDA Thailand (Food and Drug Administration Thailand). Modaplas® is an outcome of the East-meet-west knowledge integration produced by Sriracha Moda Co., Ltd. Other than blood, parts of the crocodile/alligator bodies can be effective health products. For example, dried meat, tail skin, bone and cartilage are consumed as health food in China, and other Asian countries; Australia, the Netherlands and Malaysia [AP1]produce crocodile oil and balm for sale as external medicine or cosmetics. It shows that there is still potential for development and investment in crocodile products rather than singularly skins in the future. These potential benefits can also be applied in the justification for the preservation of these species (Ross 1992). References Li, Shizhen. (1578) Compendium of Materia Medica. Trans. Xiwen Luo. (2003) Beijing: Foreign Languages Press, 4397p. Britton, A., Diamond, G., Laube, D., and Kaiser, V. (2002) Antimicrobial activity in the blood of saltwater crocodiles, Crocodylus porosus. Crocodiles: Proceedings of the 16th Working Meeting of the Crocodile Specialist Group. Gainesville, United States 7-10 October 2002. Ferguson, R., Noble, R.C., and McCartney R. (1992) Lipid and fatty acid compositional differences between eggs of wild and captive breeding alligators (Alligator mississippiensis): an association with reduced hatchability. Crocodile: Proceedings of the 11th Working Meeting of the Crocodile Specialist Group. Victoria Falls, Zimbabwe 2-7 August 1992. Buthelezi S., Southway, C., Govinden, U., Bodenstein, J,, and du Toit, K. (2012) An investigation of the antimicrobial and anti-inflammatory activities of crocodile oil. Journal of Ethnopharmacology 43(1): 325-330. Ross, J.P. 1992. Medicinal use of Crocodilians. Species (Newsletter of the Species Survival Commission) IUCN- The World Conservation Union. No. 19: 49 Merchant, M.E. Roche, C.,
Elsey, R.M., and
Prudhomme, J. (2003) Antibacterial properties of serum
from the American alligator (Alligator Mississippiensis). Comp. Biochem.
Physiol. B. Biochem. Mol. Biol. 136:505-513. Merchant M.E., Pallansch, M., Paulman, R.L., Wells, J.B., Nalca, A.,
Ptak, R. (2005).
Antiviral activity of serum from the American alligator (Alligator
mississippiensis).
Antiviral Res. 66: 35-38. Preecharam, S. Jeranaipreprem,
P., Daduang, S., Temsiripong, Y., Somdee, T., Tamo, F., Svasti, J., Thammasirirak, S. (2010) Isolation and Characterisation of
Crocosin, an antibacterial compound from crocodile (Crocodylus siamensis) plasma. Animal Science Journal 81, 393-401. Pata, S., Yaraksa, N., Daduang,
S., Temsiripong, Y., Svasti, J., Araki, T., Thammasirirak, S. (2011)
Characterization of the novel antibacterial peptide Leucrosin from crocodile (Crocodylus
siamensis) white blood cell extract. Developmental and
comparative immunology 35(5), 545-553. Links: Compendium of Materia Medica-- www.unesco.org/new/en/communication-and-information/flagship-project-activities/memory-of-the-world/register/full-list-of-registered-heritage/registered-heritage-page-1/ben-cao-gang-mu-compendium-of-materia-medica/ Modaplas®-- http://www.srirachamoda.com/modaplas/ Sriracha Moda Co., Ltd. --http://www.srirachamoda.com FDA Thailand-- http://www.fda.moph.go.th/eng/index.stm [AP1]Is
there a citation from this? I’ve never heard of the Dutch producing crocodile
oil |

 Email CSG
Email CSG