CCBM Section 44. PRINCIPLES OF CONSERVATION AND MANAGEMENT 4.1. Basic Crocodilian Biology (Perran Ross) The management, conservation and use of crocodilians is firmly grounded in the understanding of their unique biology. Details can be found in numerous standard sources in literature (eg Webb et al. 1987; Ross C.A. 1989; Ross J.P. 1998; Richardson et al. 2002; Grigg and Kirshner 2015) and on-line (www.iucncsg.org, www.crocodilian.com). Important components of crocodilian biology are the following: Ecology Large size. Crocodilians are generally among the largest organisms in their ecosystem. Crocodilians increase in size from hatchling to adult across several orders of magnitude (more than most other organisms). (Woodward et al. 1995; Whitaker and Whitaker 2008). http://madrascrocbank.blogspot.com/2008/08/worlds-biggest-crocodiles.html). This allows them to occupy different niches at different life stages and buffers larger individuals from predation by all except the largest predators and each other. Long lived. Crocodiles generally take a decade or so to reach reproductive maturity and may live several decades after that. Records from captive and wild animals indicate a potential lifespan of 50-70 years is not unusual (refs). Aquatic/amphibious habitat. Crocodilians occupy numerous different kinds of wetland and can pass over land between water bodies in response to changing conditions (eg Campos xxxx; Kofron 1993). All crocodilians need water for breeding. Diet unspecialized in size and type. Crocodilians prey upon and eat a wide variety of organisms, increasing the size and diversity of their diet as they grow older. However, even large crocodilians can subsist on surprisingly small prey as long as it is abundant (Somaweera et al. 2013). Physiology Circulation and cardiac. Crocodilians display a morphology of the circulatory system, heart and lungs that is unique among vertebrates. Their heart is 4-chambered, but allows mixing of venous and arterial blood under conditions of anoxia (Franklin and Axelsson 2000). They control heart rate and peripheral circulation, giving them good control over both oxygen consumption and heat gain and loss. Thermoregulatory and metabolic capacity. Crocodilians can maintain their internal body temperature at relatively stable and preferred levels by moving between environments of different temperature and radiant (sun) input. Their large size and circulatory structure enables them to maintain body temperatures over time and elevated temperatures enhance their physiological and muscular performance. However, they can also abandon thermoregulatory behavior, conform to ambient (and low) temperature and therefore reduce metabolic rate - greatly reducing energy use and increasing tolerance of starvation under adverse conditions (Lang 1987). Immune response and wound healing. Crocodilians have a powerful innate immune system that enables them to manage bacterial and other antigen challenges (Merchant et al. 2003). Their blood has antibiotic and antiviral properties (Merchant et al. 2005) and they have a very effective wound healing capacity, often surviving serious injuries such as limb loss. Reproduction Large clutch. Crocodilians lay relatively large clutches of 10-60 eggs (Thorbjarnarson 1996) each time they reproduce. Crocodilian females reproduce at intervals of 1-3 years or more. Older, larger females may lay larger clutches than younger smaller females. Multiparous. Females continue to nest throughout their lives, often for several decades, resulting in numerous offspring and a high reproductive output. Maternal care. All crocodilian species that have been examined show some level of maternal care of nests and of hatchlings for months up to a couple of years after hatching. There are indications that males may also participate in hatchling care. This results in a greatly increased rate of hatch success compared to other large reptiles and increased survival of young for the first year or two of their life (REF)?. Mating- multiple paternity. Most crocodilians that have been studied demonstrate complex breeding behavior prior to mating that may allow evaluation of mate fitness (Vliet 1989). Genetic studies indicate that a female may mate with several males in a season, increasing the genetic diversity of her offspring (Davis et al. 2001). Behavior Social structure. Crocodilians appear to form and maintain quite complex social interactions with each other, minimizing conflict and apportioning resources to dominant individuals (Garrick and Lang 1987a; Lang 1987b.; Seebacker and Grigg 1997). Communication by vocalization may play a part in social structure and behavior (Britton 1995). Learning capacity. Many observers in the wild, and increasing numbers of observations and experiments with crocodiles in captivity, suggest that crocodilians can learn in response to events in their environment. Behaviors including avoidance of hunters and researchers, recognition of individual humans, numerous reports of 'tame' crocodiles and successful operant conditioning to make specimens easier to handle in captivity, all suggest that crocodilians can learn and change their behavior. In combination, these features result in a highly robust life history strategy that enhances the survival of individuals, increases reproductive capacity and ensures long term survival and rapid recovery of depleted populations. These features are shared in general by all crocodilian species, but vary between species. Understanding particular species biology is therefore a key element in understanding their success, managing their populations and regulating use at sustainable levels. It is also key in identifying their vulnerabilities to natural and anthropogenic changes. Details of the application of these considerations can be found in the following sections: References Britton, A. (1995). Crocodile talk. Crocodile Communication. Crocodilians Natural History and Conservation. http://crocodilian.com/cnhc/croccomm.html Accessed 22 August 2014 Davis, L.M., Glenn, T.C., Elsey, R.M., Dessauer, H.C. and Sawyer, R.H. (2001). Multiple paternity and mating patterns in the American alligator, Alligator mississippiensis. Molecular Ecology 10(4): 1011-1024. Franklin, C.E. and Axelsson, M. (2000). Physiology: An actively controlled heart valve. Nature 406: 847-848. Garrick, L. and Lang, J. (1978). Social; signals and behaviors of adult alligators and crocodiles. American Zoologist 17: 225-239 Grigg, G.C. and Kirshner, D.S. (2015). Biology and Evolution of Crocodylians. CSIRO Publishing: Melbourne, Australia. Grigg G. (2014). Crocodile: the Largest Reptile. Australian Natural History Series, CSIRO. ISBN-13: 9780643093447 Kofron C.E. (1993). Behavior of Nile crocodiles in a seasonal river in Zimbabwe. Copeia 1993(2): 463-469. Lang J. (1987a). Crocodilian thermal selection. In Webb et al. 1987 op. cit. Lang J. (1987b). Crocodilian behaviour; implications for management. In Webb et al. 1987 op. cit. Merchant M., Roche, C., Elsey, R.M. and Prudhomme, J. (2003). Antibacterial properties of serum from the American alligator (Alligator mississippiensis) Comparative Biochemistry and Physiology Part B 136: 505-513. Merchant, M., M. Pallansch, R. Paulman, J. Wells, A. Nalca, and R. Ptak. (2005). Antiviral activity of serum from the American alligator (Alligator mississippiensis). Antiviral Research 66(1): 35-38. Richardson, K. C., Webb, G.J.W. and Manolis, S.C. (2002). Crocodiles:
Inside Out. A guide to the crocodilians and their functional
morphology. Surrey Beatty & Sons: Chipping Norton, Australia. Ross, C.A. (ed.) (1989). Crocodiles and Alligators. Golden Press Pty Ltd.: Australia. Ross, J.P. (ed.) 1998. Crocodiles: Status Survey and Conservation Action Plan 2nd edition. IUCN/SSC Crocodile Specialist Group, IUCN: Gland Switzerland and Cambridge UK viii +96 P. Seebacher, F. and Grigg, G.C. (1997). Patterns of body temperature in wild freshwater crocodiles, Crocodylus johnstoni: Thermoregulation versus thermoconformity, seasonal acclimatization and the effect of social interactions. Copeia 1997(3): 549-557. Somaweera, R., Brien, M. and Shine, R. (2013). The role of predation in shaping crocodilian natural history. Herpetological Monographs 27(1): 23-51.
Thorbjarnarson, J.B. (1996). The reproductive characteristics of the order Crocodylia. Herpetologica 52(1): 8-24 Vliet, K. (1989). Social displays of the American alligator (Alligator mississippiensis). American Zoologist 29: 1019-1031. Webb, G.J.W, Manolis, S.C. and Whitehead, P.J. (eds.) (1987). Wildlife Management: Crocodiles and Alligators. Surrey Beatty & Sons: Chipping
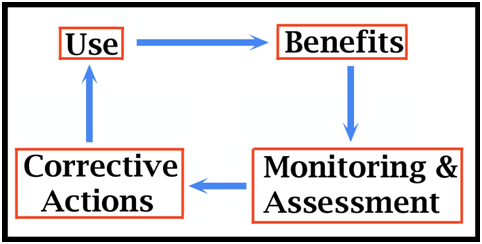
Norton, Australia. Whitaker & Whitaker 2002/2008 Woodward A.R., White, J.H. and Linda, S.B. (1995). Maximum size of the alligator (Alligator mississipiensis). Journal of Herpetology 29(4): 507-513. 4.2. Principles of Sustainable Use (Grahame Webb) This paper discusses use, sustainability and sustainable use, which for crocodilians and wildlife in general can be defined as: use of wildlife associated with a process aimed at ensuring use can continue indefinitely and that any adverse impacts are contained within defined limits. The essence of achieving sustainable use is that the harvest is matched to the intrinsic ability of the wild population to grow and replace the harvest (dynamic populations) in an acceptable time frame. When populations are reduced by harvests or other forms of mortality, changes occur (density-dependent adjustments) in separate population dynamics such as rates of mortality and rates of reproduction, allowing the population to grow. The ability to model populations and predict optimal harvest rates before harvest data are gathered is constrained by a paucity of information on the dynamic nature of the process, which varies within and between species. Despite this, many different forms of crocodilian harvest are now taking place, and some generalisations are possible. Ranching (collecting wild eggs and or juveniles for sale or for raising in farms) appears highly sustainable because only a very minor part of the population biomass is taken annually. Wild harvests of up to 1-2% of a population (which would allow trophy- or sport-hunting) have a high probability of being sustained. As harvest levels increase, to perhaps 5-10% of a wild crocodilian population, so a sustainability process (monitoring, assessment and corrective actions), within an adaptive management framework, is the best way forward. The life stages subject to harvest, for example male or female biased harvests, seems important. Experimental harvests, based on the best available knowledge, is arguably the most expedient way of gaining new and definitive information on a population’s ability to sustain harvests. Decades of research on separate population dynamics, in unharvested populations that are increasing or stable, cannot provide the information on population responses to harvest needed to inform models. Introduction A wildlife population is intended to manage needs to be described by geographic boundaries at a given point in time. The boundaries of the management area may be biologically limiting physical ones (mountains, oceans, deserts), jurisdictional ones (national borders, economic zones, land tenure allocations), or of practical or political significance (that swamp but not that river). Within a defined management area, crocodiles are usually permanent residents rather than seasonal migrants. In the same management area, there will also be human presence, with physical and jurisdictional boundaries. People may be permanent or temporary residents, and exist at different densities, potentially competing with crocodilians for the wetlands and swamps they occupy in part or the whole of the management area. From area to area, the way humans interact with crocodilians will depend on: culture, tradition, education, religion, wealth, legal frameworks, political stability, gratuity obligations, access to technology and a host of other factors. Every case history involving harvest will involve a different array of potentially interacting biological and human variables, none of which are truly stable over time. Programs need to be tailored to the context in which they are expected to work, and they need to be capable of being adapted over time if and when key variables change or drift, in response to the harvest or other factors. Problems with so many potential but unstable variables meet all the criteria for being considered wicked problems (Rittel and Webber 1973; ARCHIVE 2007; ac4d 2014). A reductionist approach to solving such problems, which attempts to quantify all the potential variables and the ways in which they integrate with each other, is unlikely to be achievable or result in predictions that can be implemented with confidence. Indeed, of the many crocodilian use programs currently operating in different parts of the world, relatively few if any are based on sophisticated, theoretical models. Management protocols tend to be “best guess” hypotheses, sometimes informed by basic models, but include some sort of sustainability process allowing corrections to the management program if something goes wrong. The lessons learned from comparing some of these practical programs are important ones for guiding new initiatives (Jenkins et al. 2006). The principles of sustainable use discussed here are simplified, hopefully user-friendly, and provide something of an introduction to crocodilian population processes. Crocodilians are predators on people and livestock, and knee-jerk political reactions to manage them in this or that way, often occur with little warning. Managers responsible for implementing these political directions may or may not have a comprehensive knowledge-base on crocodilians to help them, and may or may not have established human and resource capacity - but they need to start somewhere. What is Wildlife Use? Use is a process through which people employ something for some purpose. Hence wildlife use, and specifically uses of crocodilians, is: the processes through which people employ crocodilians for some purpose. Uses can be both consumptive or non-consumptive, although only consumptive uses are dealt with here. They can be driven by subsistence needs, economic needs or various combinations of both. The purposes of use are immensely variable and context specific. The purpose of use has little to do with the technical challenges of sustaining the use, although it may become a social issue in its own right. What is Sustainable Use?To sustain the use of anything over which people can exert control means to keep the use going over time. People have a long experience with sustaining uses, although it is not always easy. For many, sustaining families with food, shelter, medicines, security and education is a daily challenge. For the more socially advantaged, over and above basic human needs there are now a multitude of technological innovations upon which their life depends, all of which need to be continued. The approaches we take to achieving sustainable use of just about anything that provides us with benefits - the sustainability process - is basically the same: it involves monitoring, assessment and corrective actions (see below). Sustainable uses of crocodilian populations are uses associated with a process aimed at ensuring the uses can continue indefinitely. Depending on the extent of use the sustainability process may or may not require a significant investment of time and resources. Obviously, the net benefits derived from sustaining uses of crocodilians need to outweigh the costs of ensuring sustainability is achieved. The potential adverse impacts of use on other systems needs to be considered because some actions taken can undermine the ability to sustain other uses, of other resources, by people. These are so case-specific that it is impossible to give specific guidance on them. But a working definition for the sustainable use of crocodilians, that recognises the need to be mindful of broader impacts, is: use of crocodilians associated with a process aimed at ensuring the use can continue indefinitely, and that any adverse impacts can be contained within defined limits (Hutton et al. 2002; Hutton and Leader-Williams 2003). Involving Local People People living with a crocodilian resource, who are often bearing the costs of crocodilian predation on people and livestock, and other problems they cause (eg damaging fishing nets and eating fish) need to be primary beneficiaries of any commercial uses that take place. If they are not willing partners in conserving the resource, so it can be used sustainably for their benefit, then all the local incentives favour eradication. Maximising the numbers of beneficiaries amongst local people, and increasing the share of the benefits they receive, helps create incentives for them to become active partners in sustaining the uses - incentive-driven conservation (Webb 2014). The Costs of Achieving Sustainable UseThere is no standard mechanism, investment nor technical approach that needs to be applied blindly to any form of crocodilian use to ensure it is sustained. There is no mandatory requirement that all uses invest in a sustainable use process. The need for such a process will be determined by the risk that any proposed use will be unsustainable. This risk will be largely governed by how much of the population is planned to use. Opportunistic uses of wildlife by people, including crocodilians, may involve trivial amounts of the total wild population taken each year - about 0.01% (1 in 10,000). Such uses simply could not contribute to any population decline, and do not require efforts to prove sustainability. Indeed, with crocodilians, it can simply be assumed that if less than 0.5% of a wild population (1 in 200) is taken each year, and this is the only use taking place, then no measurable risk of unsustainable use may exist (Webb 2014). If any wildlife population could not withstand natural mortality rates being increased by 0.5%, they would have gone extinct long ago. However, national economic goals often seek to maximise the use of a wild renewable resource, for economic reasons. Natural renewable resources are the building blocks of economic development in many countries. As higher and higher harvest levels are planned or implemented, so the risks of surpassing sustainable levels increases, and more investment needs to be planned to ensure sustainable use is achieved. There are thus management benefits but economic costs in having conservative harvests, and economic benefits but potential conservation costs in trying to extract maximum harvests. Harvest experience and theory is perhaps most advanced in fisheries management (eg Hilborn and Walters 1992). The aim is usually to harvest the maximum amount of a resource that can be sustained, ideally with a safety margin. Nevertheless, overfishing occurs often, where the wild population declines, and reduces the amount that can be extracted annually. A declining wild population, supporting a reduced harvest, does not herald impending “extinction”. In a managed population, it can and should be a temporary aberration. More fish could be harvested annually if the fishing pressure was reduced and the wild population allowed to grow. This is not always easy to achieve, in some contexts. Overharvests are in reality the most accurate and precise way of defining what the maximum sustainable harvest or yield (MSY) really is, allowing the predictions of fisheries models to be ground-truthed against real data. What is the Sustainability Process?The sustainability process is essentially the same in fisheries, forestry or any other use of a living, renewable resources. As alluded to above, it is the same process humans use for sustaining every other dynamic, social or mechanical process they engage with (Fig. 1). The process accepts from the start that regardless of how much knowledge is available, it is impossible to predict accurately what the future may bring (a wicked problem). The sustainability process continually checks that uses are indeed being sustained. If monitoring results indicate something is starting to go wrong, it triggers further investigation, and if confirmed, triggers corrective actions. We learn more about sustainability when things go wrong than when they appear to go right. For example, if a population of crocodilians is sustaining a harvest of 5% per year, we do not know whether it could equally sustain a harvest of 6%, 7%, 8% or 9% unless we test it. Figure 1. The fundamental elements of any process aimed at sustaining anything. Use generates benefits, and Monitoring & Assessment at a level matched to the risk of unsustainable use, dictate where Corrective Actions may be needed to ensure the use is sustainable and can continue to generate benefits. A sustainable use program in a given context will always be a compromise between suites of variables broadly grouped as economic, social (including political) and biological. Because these are all changing continually but at different rates, sometimes in response to the uses, the ideal compromise (yellow dot on Fig. 2) may need to be altered from time to time. However, totally unpredictable external variables can stress any system in unpredictable ways. For example, a serious increase or decrease in demand, or the introduction of significant illegal harvest. The sustainability process simply accepts the highly dynamic, multivariate nature of most systems, and the potential need to adapt to changed circumstances. It is thus the essence of precaution.
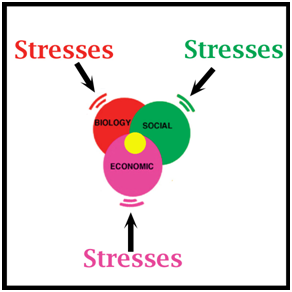
Figure 2. All sustainable use programs are compromises (yellow dot), with internal changes and external pressures (stresses) occurring continually, impacting on the sustainability of uses and benefits derived from them. Simplifying Population Dynamics in the Context of a Wild Population As discussed above, the context within which humans, wildlife and human use of wildlife all occur may be simple or complex (Fig. 3). But regardless of how complex, the essential elements needed for management can usually be simplified by using the analogy of a farm (Fig. 4). Populations of farm animals are only populations of wild animals domesticated within the last 10,000 years. The principles of managing farm animals are the same as those for managing wild animals.
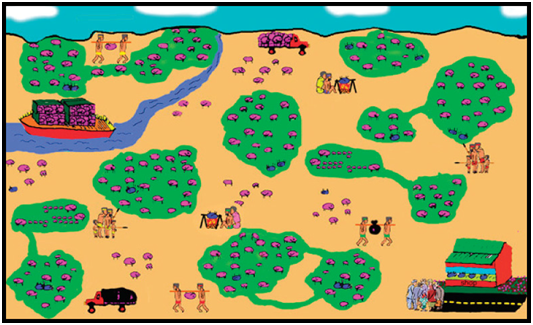
Figure 3. The context within which humans, wildlife and wildlife use by people occurs is invariably complex.
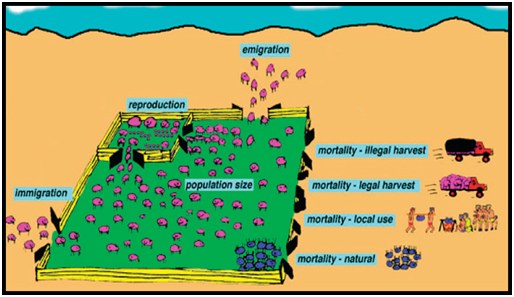
Figure 4. The situation depicted in Figure 3 interpreted in terms of a farm analogy.
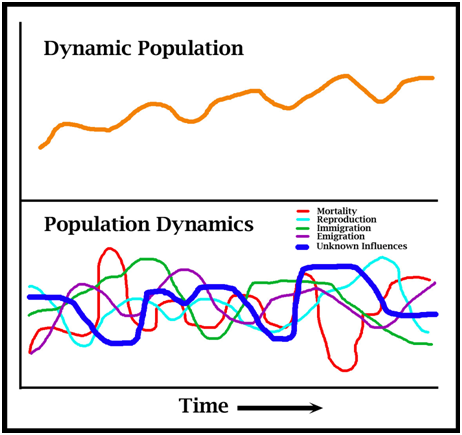
In the farm analogy (Fig. 4), the boundary of the area in which a wild population is managed is depicted by a fence. There is immigration into the management area and emigration out of it. There is recruitment through reproduction, and losses due to natural mortality, and to other sources of mortality linked to human use – local use, legal harvest and illegal harvest. In a farm, just as in a wild population, the productivity will be linked to the control exerted over the extent of uses. The size of the population can be measured, and trends in the population over time (increasing, decreasing or stable) can be determined by repeated measures. If the sustainability process (Fig. 1) is applied to such a model population, the process would be: 1. Use is taking place (legal and illegal). 2. The uses generating benefits to the owners will be encouraged and the uses not generating benefits will be curtailed. 3. The productivity of the land will change from time to time, dictating the optimal size of the population, which may need to be achieved through active intervention. 4. Monitoring abundance and population structure (juveniles versus adults) will be critical for establishing population status: whether the population is increasing (sustainable), stable (sustainable but perhaps not ideal or optimal) or decreasing (unsustainable). 5. Assessment of the monitoring results dictates current and future harvest levels, and allows any anomalies (eg new routes of illegal harvest) to be detected. 6. Corrective actions can be taken continually to maximise the sustainable harvest. What Makes Populations Grow and Decline? Without any human intervention, wildlife populations, including crocodilians, function in basically the same way. There are factors (population dynamics) that are continually increasing a wild population through recruitment, mainly reproduction and immigration, working side by side with factors decreasing a population, mainly mortality and emigration. The balance between these dynamics, in any given context, ultimately determines whether a population increases, decreases or remains stable. The population dynamics are not fixed entities - they vary in interactive ways with age, size, sex, density, resource availability and other factors (discussed below). Population Dynamics versus Dynamic Populations Biologists spend a great deal
of time and energy addressing natural history traits – it is what field biology
is all about. In the hands of those interested in population processes, this
knowledge provides insights into the main population
dynamics, which interactively determine what the population is doing.
However, the focus on separate rates (immigration, emigration, reproduction and mortality) sometimes ignores the fact that growth of wildlife populations is itself intrinsically dynamic. The concept of a dynamic population means that the population itself functions like a single organism. When depleted below carrying capacity in an otherwise stable environment a dynamic population favours population increases over decreases, promoting population recovery. If they exceed carrying capacity in an otherwise stable environment, a dynamic population will favour population decreases until some optimal level scaled to available resources is reached.
Figure 5. Separate population dynamics (lower) vary and interact with each other, and often with unknown other factors, to allow dynamic populations to respond intrinsically to depletion by increasing, and to overabundance by decreasing.
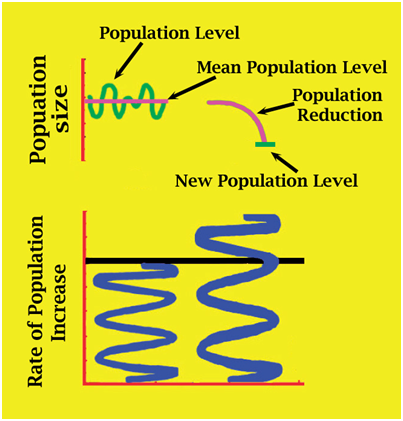
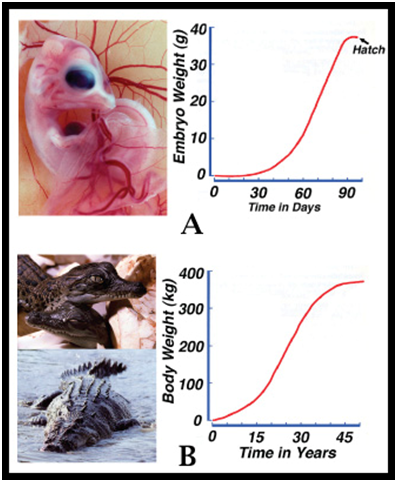
The changes that take place in a dynamic population are the result of the separate population dynamics rearranging themselves, but trying to decipher the separate roles each plays may be complex and error-prone. A potentially large number of variables contributing to changes in the population rate of increase may be unknown (Fig. 5). We can quantify what a population is doing (increasing, decreasing or stable) and through correlation can often identify why it is doing it, but knowing exactly how it is doing it may be elusive. The recovery ability of dynamic populations has been likened to a spring (Fig. 5). When the population is at carrying capacity, the spring is compressed and prevented from expanding by the population dynamics reducing the population (Fig. 6). But when the population itself is reduced below carrying capacity, the recovery spring expands, surpasses the factors reducing the population, and population expansion (recovery) occurs. Figure 6. Wildlife populations are dynamic entities. When reduced below carrying capacity the rate of population increase expands like a spring, favouring population recovery (eg Hilborn and Walters 1992). To understand how a dynamic population operates, its response to depletion needs to be studied. This can sometimes be achieved as part of a conservation program, where a depleted population is being recovered (Webb and Manolis 1992), or it can be achieved by experimental population reductions (Kennett et al. 2014). To understand how population dynamics change with changing abundance (density-dependent effects), they ideally need to be measured before, during and after an experimental population reduction (Kennett et al. 2014). Recovery Patterns – the “J-curve” As emphasized above, populations grow in biomass in the same way embryos and individual animals grow. Embryos within an egg grow until physically constrained by the shell and physiologically constrained by the inability to exchange metabolic gases through pores in it (Fig. 7A) - they then hatch. Hatchlings, unfettered from the constraints of the eggshell, grow until food or physiological systems (eg cardiovascular) become limiting – the physiological systems are not capable of supporting unlimited growth (Fig. 7B) (Caughley and Gunn 1996). Depleted populations, not restrained by resources, grow until the resources or other factors limit them at carrying capacity (Fig. 8). Despite the different time scales on Figures 7A, 7B and 8, the pattern of growth is the same logistic curve (J-curve): a slow rate of increase, followed by a high rate of increase, followed by a slow rate of increase as the population asymptote is reached (Fig. 9).
Figure 7. Growth as a function of time for an embryo, where the egg eventually constrained growth (A), and for a hatchling (B), where the physiological system eventually constrains growth (if other environmental factors do not) (Webb et al. 2006).
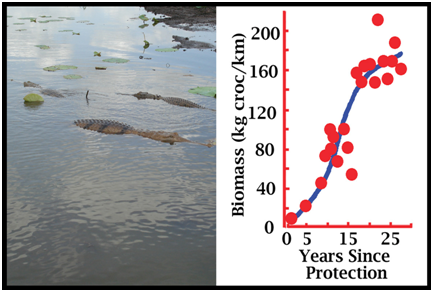
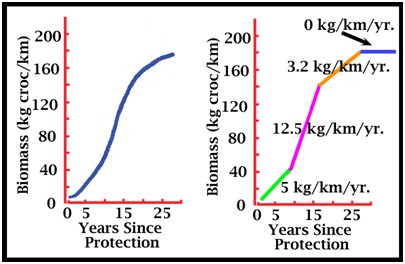
Figure 8. Growth as a function of time for a recovering population of crocodiles (saltwater crocodiles) (Webb et al. 2006).
Figure 9. The population recovery curve from Figure 8, simplified to demonstrate the rates of recovery over different time intervals, and assuming full recovery (carrying capacity; an asymptote) has been reached with zero rate of increase.
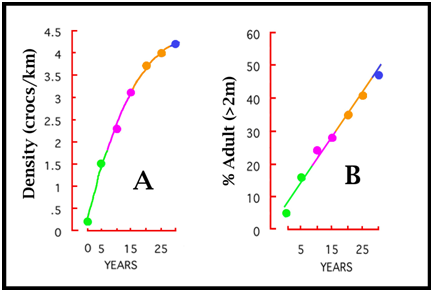
Figure 10. The recovery of the same population in Figure 9 as measured by abundance (A; crocodiles sighted per kilometre) and the changing population size and age-structure (B). Colours refer to rates of increase on Figure 9. To facilitate comparison, the index of population size in Figures 7-9 is biomass. But for the same saltwater crocodile population used to generate Figures 8 and 9, abundance and the population structure are known and have changed, but in different ways (Fig. 10). As discussed below, sex has been an important variable in some harvest strategies. As a population dynamic, sex ratio is complicated in crocodilians because embryos have temperature-dependent sex determination (TSD), and the hatchling cohort can be significantly biased from equality at the time of hatching (Webb et al. 1987, 2006). Added to this, there is an interaction between sex and size. Males grow faster than females and the asymptote is different (Webb and Smith 1984; Webb et al. 1978). For example with saltwater crocodiles in northern Australia the mean asymptote for females is about 142 kg (3.2 m TL), whereas for males the mean asymptote may be closer to 427 kg (4.8 m TL) (Webb and Messel 1978). They may reach it at the same time, but with completely different growth trajectories. This means a bottleneck of females develops in the 2-3 m TL size class, as few females pass through it, whereas all males pass through it. As a consequence, the >3 m TL size class with this species contains almost exclusively males. The younger/smaller size classes cannot be assumed to have a 1:1 sex ratio either, because of TSD on the one hand and differential sex-specific growth on the other. Strategies for the Sustainable Harvest of Crocodilians a. Basic Theory: Drawing down a population to maximise the population’s rate of growthIn theory, efforts to maximise the sustainable harvest of a wildlife or fisheries population at carrying capacity involve two stages. The first is drawing down (reducing) the population to a level at which the intrinsic rate of increase in the population is maximised. The second stage is to implement a harvest, usually annual, matched to the population increase that occurs now that the rate of population growth has been maximised (because of the draw down). With crocodilians, harvests based on deliberately drawing down a population at carrying capacity have not been implemented. But the reason is that most harvests were introduced while the population was recovering, and thus while it was probably within the area where the maximum rate of population increase was occurring. So the carrying capacity in the absence of harvest is rarely known. If we assume the population on Figure 9 had reached carrying capacity, and the drawn down model was applied, it would mean reducing the population to around 120 kg/croc/km, and harvesting the rate of increase each year (12.5 kg/km/yr). If the drawdown and harvest were random across the population structure, it would mean reducing the population from around 4.3 to 3.0 crocs/km (Fig. 10A), while maintaining the population structure at 30% adults (or crocs >2 m)(Fig. 10B). Clearly, if a harvest of 6kg/km/year rather than 12kg/km/yr (half the rate of increase) (Fig. 9) was introduced to the recovering population when it reached 120 kg/km/yr (Fig. 9), the wild population would continue to expand while the harvest was taking place. If one management priority is to rebuild a wild population, but another management priority is to create tangible incentives for people to tolerate building crocodilian populations through commercial harvest, both can be achieved in a recovering populations as long as the rate of harvest is less than the intrinsic rate of population increase. b. Ranching Eggs and Juveniles One of the most common forms of sustainable harvest of wild crocodilian populations is ranching: the collection of wild eggs and/or hatchlings and/or juveniles, for subsequent rearing on farms (Jenkins et al. 2006). The hypothesis that ranching would have a minor impact on the population (as long as adults were not being harvested simultaneously) was based on the logic that there were many surplus eggs and hatchlings, because few (maybe 1 in 1000) reached adulthood (Hollands 1987). Opposition to this logic is based on the assumption that populations are not dynamic: if 1000 eggs are required to produce one adult (due to high morality), then if 80% of eggs were harvested, the number of adults ultimately produced would be reduced by 80%. This argument is naïve because it assumes fixed population dynamics, without any density-dependent adjustments. When eggs and juveniles are harvested, the population dynamics change. In an assessment with saltwater crocodiles, hatchling survival rates from year to year increased as the number of hatchlings produced in a river decreased (Webb and Manolis 1992). So adjustments or compensation for an egg harvest (which is just an added form of egg mortality) could be made naturally within the first year. If density-dependent adjustments are ignored, all harvests will be predicted as reducing the population, with harvesting adults reducing it much more than harvesting eggs. If the non-dynamic hypothesis is accepted anyway, the harvest of eggs in a ranching program could be compensated for by releasing some raised juveniles. For example, if egg mortality rate is 75%, then the 1000 eggs collected could be expected to have produced 250 hatchlings if not collected. If hatchling mortality in the wild to two years (say to 1 m TL animals) was 80%, then by releasing 50 x 1 m long animals, the impact of the harvest would theoretically be nil. Experience has shown that this would be largely cosmetic and unnecessary if the harvest is restricted to ranching (Jenkins et al. 2006), but political compromises are sometimes unavoidable: management is rarely based on science alone. If a wild harvest program for adults is occurring at the same time as a ranching program for eggs is occurring, compensation in the form of releasing raised juveniles from the ranching program is more compelling and necessary. This is now the case in Louisiana, where the harvest is extensive, successful, sustainable and well-managed (Jenkins et al. 2006; Webb and Manolis 2006). From a biomass perspective (Fig. 9) that egg harvests have minimal impact on wild crocodilian populations is not surprising. If nesting females constitute 5% of abundance in a wild population (Fukuda et al. 2011), say 10% of the population biomass, and a clutch of eggs is about 5% of female body mass, then the eggs constitute about 0.5% of the population biomass: a very small amount. So if population biomass was 120 kg/km (Fig. 9B), the eggs would constitute 0.6 kg, in a population capable of increasing at 12.5 kg/km/yr (4.8% of the rate of increase; Fig. 9B). Clearly, if every egg was harvested, over long periods of time (14-16 years to reach maturity), and there was no immigration from unharvested areas, recruitment into the adult population would eventually be compromised. However, with the ability to harvest all eggs often being unattainable, with various density-dependent factors operating on hatchling survival rates, and with monitoring programs capable of detecting any serious decline in recruitment, egg harvests through ranching are an inherently safe way of achieving a sustainable harvest. c. Harvesting larger juveniles and adultsIn contrast to eggs, the harvesting of adults and larger juveniles is more significant. For example, with 10% of biomass for adult females, and perhaps 30% for adult males, an unregulated harvest of both in the population on Figure 9 (biomass= 120 kg/km) would equate to 48 kg/km or 3.8 times the annual rate of increase. It would rapidly lead to serious decline in abundance, which is exactly what happened historically, around the world, with all species of commercially valuable crocodilians. The population in Figures 9 and 10 suffered an historical biomass depletion of around 99% between 1945 and 1971, with abundance reduced by 95% (Fukuda et al. 2011). The remaining population was comprised of a small nucleus of wary, hidden adults, and a cohort of hatchlings emanating from their successful breeding, but with few intermediate life stages due to continual harvest. With its habitats largely intact in 1971, the population retained the ability to recover when it was protected, and has now recovered almost completely (Fukuda et al. 2011). So establishing a conservative harvest rate for larger crocodiles is vital for achieving sustainability. Calculating what the ideal rate of harvest should be will vary with species, growth rates, densities and the sizes and ages at maturity. Population simulation models (see below) can help narrow the management options, but regardless of what strategy is implemented, if the harvest rate exceeds maybe 1-2% of the population, sustainability will depend on monitoring and measuring the real impact of harvesting on the population rate of increase. Nevertheless, there are some rules of thumb that can be used as starting points. In Louisiana, before the egg ranching program was introduced, a 5% harvest rate of alligators, with a harvest strategy that favoured males over females, did not compromise the rate of population increase (Joanen and McNease 1987). It was a reasonably conservative harvest level. This would be the equivalent of harvesting around 6 kg/km in the population referred to in Figures 9 and 10, which is about 48% of the population rate of increase (12.5 kg/km/yr). The population would continue to grow while the harvest was taking place. However, once again density-dependent adjustments would be expected. In Venezuela, the annual harvest of spectacled caimans did not involve any drawdown, but was targeted at Class IV sized animals (above 1.8 m TL), which were almost all males. With this scenario, the harvested populations responded by growing in abundance, relative to unharvested populations, that stabilised at a carrying capacity less that the harvested population (Velasco and Ayarzagüena 1995; Velasco et al. 2003). This response is thought to reflect a social one. By removing the cadre of larger dominant males that were limiting the wild population through social mechanisms and predation, new cohorts could expand to take their place. The adult harvest of alligators in Louisiana may also have allowed the wild population to expand beyond where it would have stabilised without harvest, for the same reason. The harvesting of adult females does not necessarily decrease nest abundance and rates of reproduction. As depleted crocodilian populations recover, it seems that an increasing cohort of females reach adult female size, but do not nest – again perhaps for social reasons. When a nesting female is removed, one from the non-breeding cohort replaces it and starts to nest. Again, density-dependent mechanisms, perhaps driven by social dominance, that change when harvesting takes place (Hines and Abercrombie 1987). d. Combining ranching and an adult harvestSince 1985, the Louisiana harvest of alligators has involved both large-scale ranching (<400,000 eggs per year) and a large annual wild harvest (<40,000 adults and large juveniles per year; biased towards males). The population is thus harvested at both ends. Modelling indicated that this strategy was likely to compromise sustainability (Taylor and Neal 1984), and so Louisiana implemented a compensation program for the egg harvest. This entails returning the equivalent of 12% of the number of eggs collected each year back to the wild as farm-raised juveniles at 1 m in length (Webb 1985). The Louisiana harvest is clearly sustainable, although it is unclear whether various forms of density-dependent adjustments are also taking place. That is, although compensation may be needed, it is unclear what would happen if the 12% was increased or decreased. e. Trophy huntingTrophy or sport hunting occurs in a number of countries and typically targets large males. The overall harvest rate may be much less than 5% of the total population, and depending on this, sustainability may not be a problem. Trophy hunting creates a potential avenue through which a high economic return (compared to the value of a skin) can be gained with less animals taken from the wild, or it can be incorporated within a broader wild harvest program. Concerns are sometimes expressed about whether the taking of larger males may have other adverse effects. The larger males may be playing a proportionally bigger role in social structuring in the population, and theoretically, if large size is genetically controlled, their selective harvesting may eventually reduce the proportion of large males in the population or indeed the ability of the population to produce large males. The degree to which large size within a given population, inhabiting the same area is genetically controlled is unclear. When growth rates of wild crocodilians have been studied, a striking feature is the high variability in growth rates between individuals. It is the fast-growing individuals, on high growth trajectories, that ultimately reach large size. So the largest specimens are not necessarily the oldest – they are fast growing individuals. Fast-growth, in turn, appears to be something of a chance event, linked to the early attainment of dominance, which can occur when food and other raising conditions are both optimal, or well below optimal. Hence the ability to attain large size in a population that contains large-sized individuals, may be widespread, but for a variety of reasons only some individuals do so. If so, the selective harvesting of large specimens may have little, if any, effect on the ability of future generations to keep producing large individuals. At least two lines of evidence support this view. In Louisiana, where the annual harvest is targeted at larger alligators, the maximum sized animal taken each year has continued to increase rather than decrease over time. The size of animals somewhat randomly harvested has remained constant over time. The crocodilian population referred to in Figures 9 and 10 was seriously depleted by hunting historically. However, the largest animals in the recovered population today occur in similar low numbers as those reported historically. There is no empirical evidence indicating that hunting adults, particularly large individuals, eventually reduces the number of large individuals in the population. There is considerable geographic variation in the mean and maximum size of the same species of crocodile in different areas (Webb 1985), so regardless of hunting, suitable large trophy animals may be located in one population but not another. The long-term impact of removing the largest adult males has not been tested empirically, but is not considered likely to be detrimental to the wild population. As stated above, it may lead to a population increase rather than decrease. f. Using models to inform sustainable harvesting of crocodiliansGlobal understanding of wildlife population processes, in practice and in theory, has advanced greatly over the last 50 years. The technologies for modelling and simulating populations, for testing theoretical interventions, and for assessing real interventions, continues to expand. Nowhere is this more advanced than in fisheries management, where despite the wild populations being difficult or impossible to survey directly, various population indices derived from harvests are used to estimate population dynamics, and the responses of the harvested population to harvest - dynamic populations. In the case of crocodilians, population models have so far provided no “silver bullet” with regard to providing estimates of potential harvest levels and their likely sustainability that could be implemented with confidence. A vast array of natural history information has been gathered on separate population processes of different species, typically within specific “study populations” that may or may not reflect the core habitats in which these species live and are managed (Smith and Webb 1985; Tucker 1995). The data are also typically gathered from protected populations, in various stages of recovery from historical depletion, often without any clear idea of what the historical population size and structure were, and thus what the population asymptote will be when it is reached by a recovering population. These scattered data have often been used to estimate various population dynamics, as simple fixed estimates at one point in time, and these estimates in turn have been incorporated into simplistic population simulation models. Virtually none of these models account for density-dependent responses in the growing populations under study, and few if any incorporate harvest samples nor the results of harvest experiments. They simulate a theoretical situation in which population dynamics are fixed and stable, rather than the dynamic one that actually exists (Fig. 5). As a consequence, when the models are used to shed light on potential harvest strategies, and levels of harvest that may be sustainable, the results could be wildly in error. The models have proved valuable in assessing the sensitivities of population processes to different population dynamics, which helps direct where future research may be best targeted. Not surprisingly, in long-lived animals, without any primary data on density-dependence, all indicate very high sensitivity to age-specific survival rates. These are perhaps the most difficult parameters to measure in wild crocodilian populations. They can be derived from the population age structure, but with crocodilians, few age-structures have ever been quantified. Crocodilian morphological age-indicators are at best error-prone, partly because crocodilians live for long periods without growing and with limited morphological change. Models based on the size- rather than age-structure can theoretically overcome these limitations. Indeed, the harvest strategy for spectacled caimans in Venezuela discerned four classes of total length, in which the last (Class IV) was almost exclusively males (Webb 1994). By restricting the harvest to Class IV individuals, and assessing the population structure in terms of the four size stages, sustainability has been demonstrated. However with larger species, the equivalent of a Class IV harvest directed at males, may suit trophy hunting, but it would produce large skins, outside the range that the market requires. So the model would need to focus on Class III animals, where a strong female bias would exist due to their slowing growth rates (a bottleneck). More sophisticated size-based models are available, and given size is often estimated during surveys, the use seems logical. However, in the absence of harvest data, the structure of crocodiles within each size-based stage, in terms of adult and sub-adult males and females, is largely impossible to determine: there is limited if any sexual dimorphism and the tendency for females to reach adult size, but be sexually inactive until a social space is made for them, simply adds to the complications. Thus although the development of models for better understanding population dynamics, and for potentially guiding harvest options, may be beneficial in directing research to the areas most in need of research, it is important that the predictive accuracy of the models be tested and refined, at least with historical recovery data, before harvest levels predicted from the models are implemented. Regardless, if the harvests are likely to be significant, then a commitment to strategic monitoring within an adaptive management framework is fundamental to achieving sustainability. References ac4d. (2014). Wicked Problems: Problems worth solving. https://www.wickedproblems.com/1_wicked_problems.php Accessed December 2014.
ARCHIVE: Tackling wicked problems: A public policy perspective http://www.apsc.gov.au/publications-and-media/archive/publications-archive/tackling-wicked-problems. Accessed December 2014.
Caughley, G. and Gunn, A. (1996). Conservation Biology in Theory and Practice. Oxford: Blackwell Science, Cambridge, MA.
Fukuda, Y., Webb, G.J.W., Manolis, S.C., Delaney, R., Letnic, M., Lindner, G. and Whitehead, P. (2011). Recovery of saltwater crocodiles following unregulated hunting in tidal rivers of the Northern Territory, Australia. The Journal of Wildlife Management 75(6): 1253-1266.
Hilborn, R. and Walters, C.J. (1992). Quantitative Fisheries Stock Assessment. Choice, Dynamics & Uncertainty. Routledge, Chapman & Hall: USA.
Hines, T.C. and Abercrombie, C.L. (1987). The management of alligators in Florida, USA. Pp. 43-57 in Wildlife Management: Crocodiles & Alligators, ed. by G.J.W. Webb, S.C. Manolis and P.J. Whitehead. Surrey Beatty & Sons: Chipping Norton, NSW.
Hollands, M. (1987). The management of crocodiles in Papua New Guinea. Pp. 73-89 in Wildlife Management: Crocodiles & Alligators, ed. by G.J.W. Webb, S.C. Manolis and P.J. Whitehead. Surrey Beatty & Sons: Chipping Norton, NSW.
Hutton, J., Ross, P. and Webb, G. (2002). Using the market to create incentives for the sustainable use of crocodilians: A review Pp. 382-399 in Crocodiles. Proceedings of the 16th Working Meeting of the IUCN-SSC Crocodile Specialist Group. IUCN: Gland, Switzerland.
Hutton, J.M. and Leader-Williams, N. (2003). Sustainable use and incentive-driven conservation: realigning human and conservation interests. Oryx 37(2): 215-226.
Jenkins, R.W.G., Jelden, D., Webb, G.J.W. and Manolis, S.C. (eds.) (2006). Review of Crocodile Ranching Programmes. Conducted for CITES by IUCN-SSC Crocodile Specialist Group. AC22 Inf. 2, www.cites.org/eng/com/AC/22/index.shtml.
Joanen, T. and L. McNease. 1987. The management of alligators in Louisiana, USA. Pp. 33-42 in Wildlife Management: Crocodiles & Alligators, ed. by G.J.W. Webb, S.C. Manolis and P.J. Whitehead. Surrey Beatty & Sons: Chipping Norton, NSW. Kennett, R., Fordham, D.A., Alacs, E., Corey, B. and Georges, A. (2014). Chelodina oblonga Gray 1841 – Northern Snake-Necked Turtle. In Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, ed. by A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson and R.A. Mittermeier. Chelonian Research Monographs No. 5, pp. 077.1–13. Rittel, H.W.J. and Webber, M.M. (1973). Dilemmas in general planning theory. Policy Sciences 4: 155-169. Smith, A.M.A. and Webb, G.J.W. (1985). Crocodylus johnstoni in the
McKinlay River area, N.T. VII. A population simulation model. Australian Wildlife Research
12: 541-554. Taylor, D. and Neal, W. (1984). Management implications of size-class frequency distributions in Louisiana alligator populations. Wildl. Soc. Bull. 12: 312-319. Tucker, A.D. (1995). Are sustainable harvest models relevant to Johnstone's crocodile? The role of population simulations in adaptive management. Pp. 151-60 in Grigg, G. C., P. Hale, and D. Lunney (eds), Conservation Through Sustainable Use of Wildlife. Centre for Conservation Biology, University of Queensland, Brisbane.
Velasco, A., Colomine, G., de Sola, R. and Villarroel, G. (2003). Effects of sustained harvests on wild populations of Caiman crocodilus crocodilus in Venezuela. Interciencia 28(9): 544-548.
Velasco, A. and Ayarzagüena, J. (1995). Situación actual de las poblaciones de baba (Caiman crocodilus) sometidos a aprovechamiento comercial en los llanos venezolanos. Publicaciones de la Asociación Amigos de Donaña 5. 71 p.
Webb, G.J.W. and Messel, H. (1978). Morphometric analysis of Crocodylus porosus from the north coast of Arnhem Land, northern Australia. Australian Journal of Zoology 26: 1-27.
Webb, G.J.W. and Smith, A.M.A. (1984). Sex ratio and survivorship in the Australian freshwater crocodile, Crocodylus johnstoni. Pp. 319-355 in The Structure, Development and Evolution of Reptiles, ed. by M.W.J. Ferguson. Academic Press: London.
Webb, G.J.W. (1985). Survey of a pristine population of freshwater crocodiles in the Liverpool River, Arnhem Land, Australia. National Geographic Society Research Report 1979: 841-852.
Webb, G.J.W. and Smith, A.M.A. (1987). Life history parameters, population dynamics and the management of crocodilians. Pp. 199-210 in Wildlife Management: Crocodiles & Alligators, ed. by G.J.W. Webb, S.C. Manolis, and P.J. Whitehead. Surrey Beatty and Sons: Chipping Norton, NSW.
Webb, G.J.W., A.M. Beal, S.C. Manolis, and K.E. Dempsey. (1987). The effects of incubation temperature on sex determination and embryonic development rate in Crocodylus johnstoni and C. porosus. Pp. 507-531 in Webb, G. J. W., S. C. Manolis, and P. J. Whitehead (eds), Wildlife Management: Crocodiles & Alligators. Surrey Beatty and Sons, Chipping Norton, NSW.
Webb, G.J.W. and S.C. Manolis, 1992. Monitoring saltwater crocodiles (Crocodylus porosus) in the Northern Territory of Australia. Pp. 404-418 in McCullough, D. R. and R. H. Barrett (eds), Wildlife 2001: Populations. Elsevier Applied Science: New York.
Webb, G.J.W. (1994). Criteria for the sustainable use of wildlife. In Proceedings of the Workshop on “Sustainable Use of Living Natural Resources”, 19th General Assembly of IUCN. Buenos Aires, Argentina, 20-21 January 1994.
Webb, G.J.W. (1995). The links between wildlife conservation and sustainable use. Pp. 15-20 in Grigg, G. C., P. Hale, and D. Lunney (eds), Conservation Through Sustainable Use of Wildlife. Centre for Conservation Biology, University of Queensland, Brisbane.
Webb, G.J.W. (1997). Sustainable use of wildlife. Australian Biologist 10(1): 3-11.
Webb, G.J.W. (2014). Wildlife Conservation: In the
Belly of the Beast. Charles Darwin University Press: Darwin, Australia. Webb, G.J.W., Brook, B., Whitehead, P. and Manolis, S.C. (2006). Harvesting and Trade in
CITES-listed Wildlife Species: The Role of Wildlife Management Principles and
Practices. Report to CITES Secretariat. Webb, G.J.W. and Manolis, S.C. (2006). Guidelines on the Harvesting and Management of Wild Crocodilian Populations and the Determination of “Detriment” within the Context of CITES. Report to CITES Secretariat. Webb, G.J.W., Messel, H., Crawford, J. and Yerbury, M. (1978). Growth rates of Crocodylus porosus (Reptilia: Crocodilia) from Arnhem Land, northern
Australia. Australian Wildlife Research 5: 385-399.
4.2.1 Socio-economic factors At one time, the conservation of wild crocodilians was pursued exclusively through the creation of wildlife refuges or sanctuaries, the imposition of strict bans on wild harvesting, and the belief that closed-cycle captive breeding was the only rational type of use. At that time commercial utilization of wild populations was regarded almost as the first step on the road to extinction. Subsequently, the concept of sustainability came into being, and in the case of many crocodilian species that were historically exploited, it became evident that rational utilization need not affect the status of the population. It was also evident that the real problem was environmental modification through deforestation, drainage of wetlands, or more recently, intensive agriculture. From that moment, "the enemy becomes a friend"; and commercial use was recognized as one of the very few effective tools against habitat loss. The challenge was to change people's attitudes towards crocodilians, and to give them more "value". Simply telling the public that crocodilians were "good"; for the environment was not enough. People needed more tangible rewards. They got these in several ways. The first step was not to deny simple facts: crocodiles are sometimes dangerous and can be a problem, so extensive educational awareness campaigns encouraged people to treat crocodiles with caution and respect. It is now evident that sustainable utilization of crocodilians through ranching or hunting, whether alone or combined with other activities, such as ecotourism, does work positively in favour of conservation. Despite the fact that, in general terms, there has always been significant objection to the idea of harvesting wildlife for conservation, and that there are many people who will argue that it does not work, actually it has shown to be highly successful in many instances. On the other hand, even those who still harbour hostility towards crocodilians, acknowledge their biological and economic importance, and would not wish to see them disappear. Such is the importance of linking conservation with people (IIED 2014). Wild product trade involves a set of complex inter-linkages between trade, local benefits, and conservation, meaning that knowing how to promote conservation and sustainable livelihoods through trade is often complicated. For example, increasing trade volumes can be positive for conservation where it increases incentives for local people to sustainably manage the resource, or it can be negative for conservation where it drives over-exploitation and species decline. Which situation a particular trade context falls into will depend on what management regime and incentives are in place (among other factors). Likewise, regulatory interventions aimed at controlling harvest can be positive for local livelihoods, helping to ensure sustainability and control illegal take, or they can be negative for local benefits, restricting options for generating income. Livelihoods is one of the issues on which CITES was recently working [see CoP16 Doc. 19 (Rev. 1)]. 4.3. Population Dynamics"All models are wrong, but some models are useful". Empirical Model-Building and Response Surfaces, George Box and Norman Draper (1987) The tree-metaphor as a crocodilian-population model. If you plan to study crocodilians over the long haul, you'll need a model of their population dynamics. This does not mean that you need to develop a fancy computer program, replete with matrices and differential equations. Instead, we use the word "model" as a shorthand way of writing "a coherent mental picture of what sort of creature a crocodilian is."; As a biologist, you may already be familiar with the ecological concept-models of "r-species"; and "K-species";. Briefly, r-species are organisms whose population dynamics maximize reproductive rates: an r-species animal grows rapidly to maturity, it produces many offspring in one or a few reproductive events, and it does not live long. By contrast, K-species are organisms that maximize parental investment in a few, high quality offspring: a K-species animal matures slowly, it produces small litters, investing heavily in each offspring, and the K-species animal typically lives for many years. If concepts like these sound familiar, then you are sufficiently conversant with ecological models. Indeed, you could probably write (or have already written) an examination-essay about how r-species are often adapted to highly changeable environments while K-species fare better in stable environments where competition between individuals is a dominant selective pressure. During our university years, we spent too much time debating whether various taxa were "r-species"; or "K-species";. Sometimes, of course, we'd agree that a given species might fall somewhere in between the two alternatives, but we seldom questioned the basic concepts because, after all, they were printed in bold type in our high-price textbooks. However, after we began to work in real-world swamps, lakes, and streams, we soon learned that crocodilians are not r-species, that they are not K-species, and that they are not in the middle between those poles. Rather, for crocodilians, the r-species/K-species model is not merely wrong; it is not even useful. And as you work with these fascinating animals, one of your tasks will be to develop a better model. We cannot tell you what your model should look like - it must be appropriate to your needs, for your species, in your place, during your time - but we can give you a hint. For your very first (and therefore, we hope, your least useful) attempt, consider a tree model for crocodilian population dynamics. Once trees mature, they typically live for a long time. They typically produce enormous numbers of seeds - typically across multiple years - and only a tiny percentage of the seeds reach adulthood. Although you'll need to improve it, the simplistic tree-model has at least some utility. Consider, for example, a scenario under which you, as a manager, will choose some segment of a crocodilian population for a sustainable harvest. You know, of course, that the life of a parental tree (a mother crocodilian) is, demographically, far more important than even a big handful of seeds (crocodilian eggs or hatchlings). So, your tree-model suggests that harvests that target "seedling"; crocodilians may be more sustainable than harvests of adults. Building and enjoying a simple model for croc population dynamics. The fact that adult (breeder) crocodilians are more valuable, demographically, than neonates is not surprising news for anybody likely to read this essay. Nevertheless, considering this modest insight illustrates how real-world population biologists think about models. For such folks, the qualitative statement of relative demographic value might be only a first step because a scientific manager might need to know how much more valuable a breeding female might be. This quantitative concern would raise questions about clutch sizes, hatching rates, neonate survival, growth rates, age at first reproduction, and so forth. Assessments of our knowledge (or ignorance) about these population processes could lead to prioritization of research-goals and then to decisions concerning the allocation of funds for fieldwork. Several years ago we were hired to write a computer model that would simulate the demographic processes of a crocodilian population. The details of this project (which are excessively boring) need not concern you now. You should note, however, that our first step in developing this model was to list the sorts of information that we would need. Our initial list included the following 8 questions:
Note that this initial list did not contain all the questions we'd eventually need to address. Also note that these initial questions are not very well thought out. Heck, some of he questions are even asking the same sort of thing. For example, the answer to question #8 involves an answer to question #7 (in addition to information about survival and longevity rates). Our point is that you do not need to begin with a perfect list, but you do need to write something down. Your list - which is, in a sense, your model - can be improved as you go along. So, when you initiate your study of wild-crocodilian population-dynamics, please begin with a need-to-know list (or even use ours if you have to). Next, for each question, please write down your numerical, best-guess answer. Then evaluate your answers: if you think your guess is pretty good, mark it with a single question-mark. If you are less confident, use two question-marks. If your answer is merely a SWAG (English acronym for "Scientific Wild-Ass Guess";), then use three question-marks. If this process generates a whole lot of question-marks, do not despair: this is just an indication that you should discuss answers with your colleagues, consult the published literature, and email any "experts"; who might write back. Throughout this talking-and-reading research, you should continue to seek numerical answers. And for each question you should write down the largest and smallest "answers"; you are receive. (This is important; like your question-marks, the variation in these answers may indicate the overall level of uncertainty). A modeler's next step would be to sit down and think systematically about this list of answers: meditate particularly on how well the answers do or do not fit together. Below we'll give you a numerical answer of what we mean. Here are some numerical answers you might have derived.
If we accept these estimates, we can calculate the number of daughters-reaching-adulthood a newly reproductive female might expect to produce. See if you can figure out how we'd do this. It's really pretty easy. (We'll call the calculated quantity RR; you can think of it as a replacement rate. Note that for a population to survive indefinitely, this replacement rate must be at least 1.0.) RR = (Answer to 8)(Answers to 1 & 2)(Answer to 3)(Answer to 4)(Answer to 5(Answer to 6-1)) RR = 6 x (30*0.4*0.5 x 0.5 x 0.2 x 0.811 = 0.31 Note that 0.31 is less than a third of the minimum RR needed to sustain a population. This means that either some estimates in our model are too small - or that we'll soon be out of a job because our population is rapidly crashing. In other words, some of our estimates for demographic parameters are not fitting realistically with others. If you appreciate the sort of exercise we illustrated in the box above, then you might also enjoy entering different parameter-estimates - perhaps your high-end and low-end guesses - into your simple arithmetical model (see below). It can be fun to see which changes make the most difference. Note, for example, that if we double the value of Parameter #1 (Average Clutch Size to 60 eggs), then we also double the value of RR, from 0.31 to 0.62, which will still not sustain our population. On the other hand, if we merely increase Parameter #5, Sub-Adult Survival, from 0.8 to 0.9, RR will consequently increase from 0.31 to 1.13, which would suggest population growth. Some modelers call this process "sensitivity testing,"; and they use it to seek insights into the relative importance of demographic parameters. We agree that sensitivity testing can be useful, but it will probably tell you more about the model you have created than about the real population you seek to model. Ask yourself why, in our example, doubling Clutch Size changes RR so little while a lesser increase in Sub-Adult Survival changes RR so much. This difference in effect does not illustrate some major insight about crocodilian biology! Instead, it reflects the model-fact that Sub-Adult Survival is actually a compounded parameter. That is, it combines "Survival from Year 1 to Year 2"; with "Survival from Year 2 to Year 3,"; and so forth until adulthood (Year 12). If we had modeled these 11 parameters separately, then, for example, a 10% increase in any one of them would have been reflected by a 10% increase in RR. We have elaborated this point because, although we are population-modelers, we understand that models themselves do not provide new information about the natural world. Instead, they are useful because they say, "Here are some consequences of what you already believe about the natural world";. If those consequences are unexpected - and sometimes they really are - then your model is a useful one! But it's still not providing new information. Very simple models, like the multiplicative example presented above, seldom tempt us to believe that they generate Truth-with-a-capital "T."; More sophisticated efforts, however, can be more seductive. Earlier in our careers we developed very complex models for populations of long-lived vertebrates. Because we worked on the models for months and because they cost lots of money, some naive field biologists, bamboozled by user-hostile equations, believed that those models could discover hitherto unknown facts about the biological world. This, of course, was not true. The models could merely organize, combine, and manipulate existing information (or assumptions). Of course the sensitivity testing we inflicted on our models did not give us New Truth either. On the other hand, this exercise did tell us, "You need to look more carefully at several aspects of crocodilian biology";. Among those aspects were:
Building "dynamics" into the model. Thus far we have written as if the numbers describing a crocodilian population's demographic processes never change, as if, for example, "Hatchling survival is now, and ever shall be, 20%";. Such, of course, is not the case. The demographic numbers most certainly can change. So call them "variables,"; not "parameters";: after all, they describe the behavior of living, complex organisms, and they reflect the interactions of a population with a changeable environment. Some demographic variation seems absolutely unpredictable. Computer modelers often call it "noise"; and simulate it by means of random-number generators. We shall not discuss such "random"; variation further, but every croc-manager should remember that real crocodilian populations are less understandable - and more interesting - than any numerical theories we can apply to them. Indeed, this "random unpredictability"; reminds us that one characteristic of a good field ecologist is an abiding modesty. By contrast, some demographic variability is directly linked to changes in external environment. Investigating the most important croc-with-environment relationships is a critical task for a population-manager, and we shall consider it, briefly, later. Finally, some demographic variation is most conveniently thought of as internal to the croc population itself. This last type of variation can be particularly important to a population-manager, and we'll illustrate it by two specific examples. Two examples of density-dependent factors. Because a population's ecological density measures the number of animals per resource, it can change as an environment changes. However, for simplicity's sake, many modelers prefer to pretend that environmental conditions are constant - while the number of animals is the density-factor that varies. And as every reader of this essay already knows, when the number of crocs in a given piece of real estate increases (or decreases) radically, the demographic processes of those crocs' population also change. Consider our demographic variable, "Average nesting frequency of adult females";. The relationship between Nesting Frequency and density may be quite complex. Let us say, for example, that nesting habitat is limited. Then, in order to nest, a female may have to accumulate sufficient nutritional resources (1) to pay the physiological expenses of egg-production, nest-building, and nest-guarding and (2) to compete against other females for the limited number of nesting sites. If a crocodilian population is increasing, the nest-site competition will become more intense (more expensive, physiologically) - perhaps at the same time that per-croc food availability is declining. Therefore, Nesting Frequency would be expected to decrease as croc-density increases. Conversely, with fewer crocs in the population, a higher percentage of females could find good places to lay their eggs, and, on average, they might catch enough food to recharge their reproductive systems in one year instead of two or three. Thus, a decline in density might be associated with an increase in Average Nesting Frequency. All you croc biologists realize that the nesting-and-density relationship is more complex than we've made it sound. If good nesting sites are limited, then females may still nest - but at less appropriate sites. In that case, density might affect Hatch Probability or Hatchling Survival rather than Nesting Frequency. Nesting Frequency may also be affected by crocodilian's social structure, which may itself be associated with density. Consider a Nesting-Frequency scenario, not entirely hypothetical, that would have important management implications. Imagine an environment with limited nesting sites. Also imagine that the environment's crocodilian population has a cohort of females that largely monopolize those nesting sites and another cohort of females that are seldom able to nest at all. Perhaps a Management Authority has established a harvest-limit for that population. Furthermore, the Management Authority has decided to monitor harvest-affects by counting crocodilian nests from the air. (Note that, under many conditions, experienced observers can make accurate nest-counts from rotary-wing aircraft. Indeed, if you can afford the helicopter time, this may be the best way to monitor nesting). During the first few harvest-years, the aerial nest-count remains constant, even though a substantial number of breeding-sized females are being removed. Then, in subsequent years, the nest-count declines in exact proportion to the number of adult females harvested. What could be happening? During the early years, if you killed a nesting female, another female from the "disadvantaged cohort"; would nest in her place. Eventually, however, every female has a chance to nest - so, when you remove one, the number of nests declines accordingly. Hatchling survival may also be affected by density. This can occur for many reasons. At high hatchling densities, food may be relatively scarce, and some baby crocs may starve while others forage less cautiously and fall victim to depredation. Conversely, at low densities, food may be so abundant that foraging-exposure to predators is reduced. Or perhaps low-density hatchlings feed ad libidum and therefore grow rapidly into safer size-classes. In some environments predators may alter their foraging behavior based on the available densities of tasty young crocodilians. This may even include conspecific predators: for several crocodilian species, as hatchling densities increase, young crocodilians are increasingly subjected to cannibalization by their elders. In the USA, introductory wildlife-management textbooks are replete with examples of density-dependent effects on reproduction, growth, and survival. Some examples focus upon prey-switching behavior of potential predators, access to shelter, or availability of suitable breeding territories. Most discussion, however, emphasizes the importance of food. For a beginning student of American wildlife management, the take-home message is clear. Recruitment to a population slows down as that population approaches a saturation density, or "carrying capacity";. Conversely, the population will increase more rapidly if it is maintained (perhaps by hunting) at a lower density - and thus there is an "exploitable surplus"; that can be harvested on a sustained-yield basis. Of course these principles do apply to crocodilian populations. We would, however, offer a precautionary warning. The exploitable-surplus idea, sacred to US game management, was first developed for endothermic species with relatively brief life-expectancies. For at least 3 reasons, crocodilians are different.
If you play around with numbers, you can demonstrate that the population-value of a reproductive female crocodilian is very high. (Given the numbers we used in our first example, that value, measured in crocodilian eggs, is almost 600). By contrast, the value of a newly matured, female whitetail deer is much lower (measured in fawn-equivalents, that value is about two orders of magnitude less!). In other words, although the basic harvest principles of traditional American game management definitely apply to crocodilians, a manager would be foolish to apply them uncritically, without consideration of the demographic realities that make crocodilian populations special. Direct environmental effects on crocodilian population dynamics. As you have certainly learned through your work with crocodilians, their population processes can be affected by numerous environmental factors. (Indeed, even the demographic changes that we modeled above as population-intrinsic can actually be traced back to environmental causes). You know that variations in temperature, prey base, or aquatic vegetation can impact growth rates, hatching probability, or even sex ratio. And you have probably read that the accumulation of pesticides, herbicides, or other chemical pollutants can affect crocodilian populations in ways that scientists are only beginning to understand. So, an enormous number of environmental variables impact crocodilians in important ways. Nevertheless, of all the environmental factors that we routinely measure, water levels can - at least in the short run - cause the most dramatic shifts in croc population structures. Depending on the environment (including, particularly, the physical shape of the hydrologic landscape), an ill-timed flood can cause the loss of an entire year's nesting effort, and an ill-timed drought can spatially compress young crocs so that entire cohorts are wiped out by cannibalism or other depredation. Therefore, wise crocodile-management biologists maintain careful records of water levels and seek to monitor their effects on managed populations. Stability amid change (or, back to the tree-model). Given all the ways in which multiple environmental factors directly affect various parameters of a crocodilian population, and given all the ways in which those effects reverberate even through demographic parameters not directly impacted, the beginning croc-biologist may wonder whether any general insights can be derived from this complex mess. We believe that there are indeed two important general lessons. The first is that complexity is interesting. As Mr. Darwin wrote long ago, "…there is a grandeur in this view of life…,"; and our mission as biologists is to affirm the grandeur, to appreciate the complexity, and to be modestly thankful that we have opportunity to study the incompletely elucidated mysteries of the Order Crocodilia. Our second important take-home message about crocodilian demography is illustrated by the tree-and-seeds model/metaphor with which we opened this essay. Throughout our careers we have observed that many things about a crocodilian population can change from one year to the next. Flooding destroys nests. Aberrant dry-season weather disrupts the growth-rates of hatchlings. A cold winter for range-marginal populations doubles the death-rates for juveniles. But, across these changes, what stays the same - or at least doesn't change very much? The answer is, the cohort of reproductive adults. As we learn more about the biology of large crocodilians, we become increasingly impressed by the physiological homeostasis of adult individuals. Typically, they don't grow much in good years, they don't lose much weight in bad years, they are almost immune from depredation, and sometimes they don't even change their nesting sites by 50 metres over the course of two or three decades! Here are two caveats about our general homeostasis-insight. First, our generalizations do not apply equally to all crocodilian species. Spectacled caiman, for example, tend to reach adulthood pretty fast, and survival rates of their adults are substantially less than 100%. And for some range-marginal populations (such as Crocodylus acutus in southern Florida, USA), severe winter weather can kill even the largest adults. Still, for some species, under many ecological conditions, survival rates for large adults are very high, and our insight of physiological homeostasis tends to prevail. (We are thinking particularly of large species such as Gharials and American alligators. However, adults of even small species sometimes manifest high survival rates over many years. Consider, for example, a population of Paleosuchus trigonotus in rainforest streams in central Amazonia). Indeed, the very largest crocodilians, living in moderate climates, hardly even change their body-core temperatures with environment; instead, they can be practically homeothermic. Second, under some density conditions (and this is particularly true for some species), agonistic social interactions can impact the large-adult cohort. Big males, for example, can and do fight, sometimes with lethal results. And even females (the more important gender for our modeling exercises) occasionally kill one another in conflicts over nesting sites - and sometimes for other reasons that we do not understand. Our point is that, like great trees, fully mature crocodilians tend to survive all but the greatest ecological "storms";. Let a flood wipe out a year's nesting: the mother crocodilian will be around to nest next year (or the year after, or the year after that, if inauspicious conditions drag on and on). Let protracted drought delay the maturation of juveniles for half a dozen years (or let bull-crocs enter the juveniles' habitat and eat them all up): the breeders will survive. Let flood or drought or shading-competition or tragedy of any sort befall the "seeds"; produced by the great tree: the tree survives, and so long as it does, the propagation of its species has at least the chance to continue. So, here is our final take-home message for managers of crocodilian populations. The homeostatic survivability of adult, breeding crocodilians gives us room to make substantial errors in our management of younger, smaller, age classes, so long as we monitor residual populations and remain willing to modify our policies once our mistakes become obvious. On the other hand, errors in the management of larger crocodilian will not repair themselves so easily, and we need to be more careful, more thoughtful, if we choose to manipulate their populations. 4.4. Management Programs Overview (Perran Ross) Management programs for crocodilians fall into two broad classes, standard conservation protection (e.g. protection, legislation prohibiting killing, protected areas, reintroduction etc.) and active management programs based upon sustainable use. The Crocodile Specialist Group has promoted sustainable use programs for those species and populations able to support regulated harvest in the belief that people will conserve what they value and derive benefit from (see preceding sections 4, 4.1, 4.2, 4.2.1 insert links). There are three ways in which crocodilians (and other species) can be sustainably exploited for commercial gain and conservation benefit: Direct harvest from the wild (section 4.4.2 link)- hunting those size classes that produce marketable products. The specialized hunting for large 'trophy' specimens (section 4.4.4 link) is a subset of this mode with rather different features. Ranching (section 4.4.1 link)- defined here as the collection of eggs or hatchlings to be raised in captive facilities. Captive breeding (section 4.4.3 link)- sometimes called 'farming' or 'closed cycle captive breeding'- where adult specimens are maintained in a facility as breeding stock and the offspring they produce in captivity are raised. These each have advantages and disadvantages in terms of the direct benefit to conservation, ease of regulation and management control, and requirements for technical, economic and husbandry resources to successfully operate. Table 1. Indication of comparative advantages and disadvantages of different modes of crocodilian production. See following sections for details.
Guidelines for the framework of a sustainable program were
drafted by CSG in 1994, David 1994 Pp. 274—in
https://www.iucncsg.org/365_docs/attachments/protarea/12th-b3e47de4.pdf
David notes (Pp 276) "The two most important elements of any sustainable wild crocodilian use program are effective trade control and technically sound monitoring. However, there is no single prescription for a program to utilize wild crocodilian resource(s); each and every region of the world has unique needs requiring a different emphasis."; In practice programs in many countries successfully and effectively combine different elements of wild harvest, ranching and captive breeding while others continue to conserve crocodilians, particularly those of low economic value, using standard conservation measures. For example wild harvest is successfully managed on a sustainable basis in Bolivia (Caiman yacare), Brazil (M. niger), Nicaragua (Caiman crocodilus), Papua New Guinea (C. novaeguineae), USA (Alligator missisipiensis), Venezuela (Caiman crocodilus), (refs). Ranching programs have been successful in Argentina (C. latirostris), Australia (C. porosus), Papua New Guinea (C. porosus) USA (A. mississipiensis), Zimbabwe (C. niloticus) Refs . Captive breeding is the preferred mode of production in China (A. sinensis, C. siamensis), Cambodia( C. siamensis), Colombia (C. crocodilus), Philippines (C. porosus), Thailand (C. siamensis), South Africa (C. niloticus) refs and a component of programs in several other countries already cited e.g. USA, Australia. Standard conservation practice is effective on endangered and low value species in several countries including China (A. sinensis), Mexico (C. acutus), India (C palustris, C. porosus and G. gangeticus) Philippines (C. mindorensis). However, it is noteworthy that although many countries with crocodilians have conservation protections, protected areas etc., without adequate funding or technical capacity and with disinterest or opposition from local people, the effectiveness of these programs is equivocal. Numerous reports of the operation, status and activities of all these programs can be found in the CSG newsletter (quarterly) and Proceedings of the Working Meetings( bi-annual) at the CSG website https://www.iucncsg.org/pages/Publications.html . 4.4.1. Principles and operation of Ranching (Alejandro Larriera) Ranching refers to the removal from the wild of juveniles or eggs, which are then transferred to controlled raising facilities, where they are grown for commercial purposes. It has been the preferred management option for crocodiles in some countries (eg Papua New Guinea) since before CITES came into force. Currently, countries such as Australia, Argentina, USA, Zimbabwe and more recently Bolivia and Brazil, are utilizing ranching as a conservation tool for their crocodilian populations. The 3rd meeting of the Conference of the Parties adopted Resolution Conf. 3.15 on 'Ranching', which provided a specific legal framework for Parties to the Convention to be able to transfer Appendix-I listed species to Appendix II, if the utilization proposed was largely restricted to ranching and there were clear conservation benefits. Resolution Conf. 3.15 was replaced by Resolution Conf. 11.16 (Rev. CoP15). This means that there are two avenues through CITES where ranching can be pursued. First, it can be a standard management option adopted by any Party, for any Appendix-II species, as long as the management satisfies Article IV of the Convention. Second, Appendix-I species can be transferred to Appendix II specifically for the purposes of ranching [Resolution Conf. 11.16 (Rev. CoP15)], and largely limited to that form of utilization, in which case additional conditions apply. Numerous Parties have sought the transfer from Appendix I to Appendix II specifically for ranching (under the original Resolution Conf. 3.15 or Resolution Conf. 11.16). The prerequisites for seeking such a transfer to Appendix II under the ranching resolutions are: (i) the population of the species is no longer considered to be endangered; and, ii) the population's continued recovery and conservation would benefit from a ranching program. Many crocodilian species have been transferred to Appendix II through Resolution Conf. 11.16 (Rev. CoP15)] (see Jenkins et al. 2004). However, changes to Resolution Conf. 9.24 (Rev. CoP15) on 'Criteria for amendment of Appendices I and II' meant that ranching proposals, in addition to satisfying Resolution Conf. 11.16 (Rev. CoP15), also had to meet the criteria of Annex I of Resolution Conf. 9.24 (Rev. CoP15) for inclusion in Appendix II. The intent of the ranching resolution, to be able to transfer an Appendix-I population to Appendix II, where that population did not meet the criteria of Resolution Conf. 9.24 (Rev. CoP15) for Appendix-II listing, but where there were clear conservation benefits to the population in doing so, was lost. More recently, the CITES Animals Committee was directed to evaluate the merit of retaining the ability to transfer to Appendix II through the ranching resolution (see CoP16 Doc. 72). 4.4.2. Principles and operation of Wild Harvest (Alvaro Velasco) Wild Harvest refers to the removal of individuals from the wild population for commercial purposes. This management option is used with different species in different countries, for example Caiman crocodilus in Guyana and Venezuela, Caiman crocodilus fuscus in Nicaragua; Caiman yacare in Bolivia; Melanosuchus niger in Brazil; Alligator mississippiensis in USA, Crocodylus niloticus in some countries in Africa; Crocodylus novaeguineae in Papua New Guinea and Crocodylus porosus in Australia. The wild harvest applies only to species on Appendix II of CITES. The basis of this management option is to crop a sustainable portion of the wild population and depends on abundance and size classes, which are determined through surveys and monitoring the population in order to define the annual harvest quota (Velasco and Ayarzaguena 1995). The portion of the population to be harvested depends on the management program. In Venezuela, the harvest is based on the larger caimans named class IV, defined as adult males with total length over 1.80 m (Velasco and De Sola 1999). The per year harvest is no more that 25% of this size class. In Brazil the section to be harvested includes males and females but is only permitted outside of breeding season, as in other countries. The controls are via mechanisms such as licenses, the size of caimans harvested, the tags and export quantities. The impact of this cropping on the population can be determined by making annual surveys on the population under the program, comparing areas with harvest vs areas without harvest (Velasco et al 2003). References Velasco, A. and Ayarzagüena, J. (1995). Situación actual de las poblaciones de baba (Caiman crocodilus) sometidas a aprovechamiento comercial en los llanos venezolanos. Publicación de la Asociación de Amigos de Doñana. N°5. 71pp. ISSN 1132-8398. Velasco, A. and De Sola, R. (1999). Programa de Manejo de la Baba
(Caiman crocodilus) de Venezuela. Vida Silvestre Neotropical.
8(1-2): 10-17. Velasco, A., Colomine, G., De Sola, R. and Villarroel, G. (2003). Effects of sustained harvest on wild populations of Caiman crocodilus crocodilus in Venezuela. Interciencia. 28(9): 544-548. 4.4.3. Principles and operation of Captive Breeding (Robbie Mcleod and John Lever)The following general principles and pointers were generated by the Koorana Crocodile Farm team based on their long experience with Saltwater crocodiles, C. porosus, and can be applied, with appropriate species specific adaptation, to other species[PR1] . 1. Obtaining Breeders
2. Breeders from wild populations
b. enclosure boundaries (i.e. fences) c. human interaction 3. Captive bred breeders Have many advantages:
4. Pair selection and matching
5. Pair introduction (1 to 1 pairing)
6. Pair introduction (colonies)
7. Pen/enclosure design as it relates to breeding soundness
8. Nutrition
9. Hygiene
10. Assisted breeding
11. Inbreeding
12. Egg collection/incubation
13. Incubation
[PR1]Robbie/John-I added this short introductory sentence- modify or add as you think best. [PR2]It would be useful to indicate a range or average time period for general guidance e.g. " between 3 and 10 weeks"; ot even some personal observations e.g. " We have seen the most aggressive pairs become habituated to each other after 10-12 weeks of such exposure."; Doesn't have to be science or a large sample- just and indication so the reader will know what to expect. [PR3]Many commercial farms allow embryos to stay 'dry' for
up to 24 hrs before introducing them to shallow water- ostensibly to
allow yolk sac resorbtion. However its pretty clear from detailed
(video) studies of nest hatching that the crocs pip their egg but do not
emerge until immediately before and duting nest opening by the mother,
who may also actively assist hatchlings to leave their egg-so your way
seems more 'natural' to me JPR 4.4.4. Principles and operation of Trophy Hunting (Brian M. Jeffery) Abstract Trophy hunting is defined as hunting by a paying client (usually accompanied by a professional guide) with the objective of harvesting individual animals with best physical attributes (e.g. body size, antler points, tusks, rarity, etc.). Trophy hunting has been used for a number of big game animals, including crocodilians and usually exerts selective pressure on adult males. The ventures are usually hunting operators who market and sell hunts on owned or leased hunting areas with essential staff (professional hunters/trackers, drivers, skinners/butchers and camp staff). In several African countries, the hunting industries and government agencies united to create programs that incentivize trophy hunting to contribute to conservation. In addition, the lease agreements usually require assistance with anti-poaching policies. Where well managed, trophy hunting involves low takes and is sustainable. Trophy hunters pay a higher fee per client than conventional tourists and revenue can be generated from lower volumes of people. With low takes and high fees, trophy hunting can play a role in conservation of threatened and endangered species. Trophy hunting can be a viable alternative to areas where conventional tourism is low. In addition, as trophy hunting is primarily motivated by the thrill of the hunt and the subsequent acquisition of a trophy, it can be carried out on land that is less scenic than that demanded for wildlife tourism. These areas may lack the infrastructure, attractive scenery, or high densities of viewable wildlife that conventional tourists come to expect. Trophy hunting revenues are vital to these areas as conventional tourism or ecotourism do not generate enough income for protection and may be more resilient than tourism in politically unstable countries. Trophy Hunting Review Trophy hunting takes place in North America, Europe, and in developing countries where wildlife management infrastructure is often less fully developed (IUCN SCC 2012). It is a form of wildlife use, when managed, may assist in promoting conservation objectives by creating revenue and economic incentives for the management and conservation of targeted species and habitat (IUCN SCC 2012). If managed poorly, it can fail to deliver these benefits. Hunts are usually conducted by a person paying a considerable amount of money for the opportunity (Lindsey et al. 2007). It typically involves taking small numbers of individuals and does not require development of an infrastructure. It is a high value, low impact type of tourism. A wide variety of species (some of which are both common and secure) are hunted for trophies. Species that are rare or threatened may be included in trophy hunting as part of site-specific conservation strategies (IUCN SCC 2012). The IUCN SSC believes trophy hunting will likely contribute to conservation and to the equitable sharing of benefits of use from natural resources when programs incorporate the following five components (IUCN SCC 2012):
While trophy hunting is a useful tool in conservation, there are factors limiting the success of the program. These include ethical, biological, and social (Lindsey et al. 2006). In some African countries, game ranchers are required by law to have perimeter game fencing. Many of these areas are small and overstocked (Lindsey et al. 2007). Exotic species are often introduced onto game ranches to increase the diversity of saleable trophies (Hamman et al. 2003; Lindsey 2005). In some cases, species are hybridized to offer unique trophy prize (Hamman et al. 2003). If these exotic and hybridized species escape to the wild, it could cause ecological problems. Some ethical issues have been raised and have a negative impact on public perception of trophy hunting. Activities such as shooting from vehicles, shooting females or immature individuals, luring animals from parks, using bait stations, hunting with dogs, put and take (releasing trophy animals immediately before a hunt), and canned hunt (shooting animals in small enclosures with no chance to escape) are under scrutiny by activist groups. In some countries, there are not enough resources to adequately census wildlife populations and quotas are based on guesswork (Caro et al. 1998). The valuation of wildlife as trophies creates pressure to issue large quotas (Lindsey et al. 2006). Moreover, many regions lack the resources to enforce existing quotas. Trophy hunting can be a lucrative business while helping to conserve wildlife and its habitat if managed properly. It incentivizes the protection of habitat and maintenance of sustainable populations in areas where there is no conservation alternative. However, poor governance, corruption, and unethical/ unsustainable practices persistently weaken its success as a conservation tool. Crocodilian examples of trophy hunts: Nile Crocodiles are hunted with guides in Tanzania, South Africa, Mozambique, Zambia, and Namibia on private reserves or concession areas. The crocodiles are hunted mostly with rifles with a minimum .300 caliber round and in blinds. Shots are only fired at individuals on dry land. American Alligators are hunted with guides in the United States in Florida, Georgia, Louisiana, South Carolina, Alabama, Mississippi, and Texas on public and private lands. Methods of take vary among states. The most common methods used are harpoon, bow and arrow, baited lines, and crossbows. Literature Cited Caro, T.M., N. Pelkey, M. Borner, E.L. Severre, K.L. Campbell, S.A. Huish, J. Ole Kumai, J.P. Farm, J.L. Woodworth. 1998. The impact of tourist hunting on large mammals in Tanzania: an initial assessment. African Journal of Ecology 36: 321-346. Hamman, K., S. Vrahimis, and H. Blom. 2003. Can current trend in the game industry be reconciled with nature conservation? In: Damm, G.(ed) African Indaba Yearbook 2003 19-38. IUCN SSC. 2012. IUCN SSC Guiding principles on trophy hunting as a tool for creating conservation incentives. Version 1.0. IUCN, Gland. Lindsey, P.A. 2005. A review of the economic contribution of the trophy hunting industry in Africa. Safari Club International Report, Safari Club International, Washington D.C. Lindsey, P.A., P.A. Roulet, and S.S. Romañach. 2007. Economic and conservation significance of the trophy hunting industry in sub-Saharan Africa. Biological Conservation. 134: 455-469. 4.5. Human-Crocodile Conflict
4.5.1. Problem Crocodile Control (Allan Woodward) The population recovery of many species of crocodilians since the 1960s and 1970s has been an endangered species success story, but it has not been without complications. This is particularly true of the larger species of crocodilians that have the propensity to attack humans or their domestic animals and damage fishing gear (Woodward and Cook 2000; Aust et al. 2009). The species that have caused most problems are the Saltwater crocodile (Crocodylus porosus), Nile crocodile (C. niloticus), American alligator (Alligator mississippiensis) and American crocodile (C. acutus). Increasing incidents of attacks on humans have been reported for the Black caiman (Melanosuchus niger), and the Mugger crocodile (C. palustris) (see CSG 2016). However, even less dangerous and smaller species of crocodilians can cause conflicts with people by preying on their livestock, competing for fish, or damaging embankments. Balancing the conservation of crocodilians with the safety and well-being of people living in close proximity to them is a challenge for natural resource management agencies. When crocodilians do pose a threat to people or their property, there is usually an expectation that the problem will be resolved in an expeditious manner. If not, attitudes of stakeholders toward crocodilians can become more negative, which can undermine broader crocodilian conservation goals. Typically, management agencies deal with problem crocodile conflict by educating the public, removing individual animals, and by reducing crocodilian populations. Education Enhancing the knowledge and awareness of people about the dangers of crocodilians, and encouraging people to avoid situations that endanger their and their animals' safety can help people live in reasonable harmony with crocodilians. Several programs have been developed to educate people about living with crocodilians, including "Be Crocwise"; (Northern Territory, Australia), "Living with Crocodiles"; (Queensland, Australia) and "Living with Alligators and Crocodiles"; (Florida, USA). Public education programs such as these can not only reduce human injuries and loss of domestic animals but can reduce funding demands for problem crocodile control by management agencies. Selective Removal of Targeted Problem Crocodilians A variety of direct and indirect capture methods are used to capture problem crocodilians (see Sections 5.1 and 5.2 of this manual). Moving problem animals into captivity: This is generally used in situations where there are adequate facilities willing to receive crocodilians and permitting can be worked out (Letnic 2004). In general, it is desirable to use facilities that meet minimum standards for crocodilian housing and care. For rare species, it is desirable to transfer them to facilities that have captive breeding programs for reintroduction. Translocation of problem animals to more remote locations: Translocation is typically used for threatened species, populations of species that are recovering from a depleted status, or crocodilians in areas where lethal control is not acceptable (Hines and Woodward 1980; Letnic 2004; Reed et al. 2004). However, translocation has met with mixed success. Some animals may remain at the recipient site but, unfortunately, most species of crocodilians have a strong homing instinct and generally attempt to return to the capture site (Murphy 1983; Rodda 1984, 1985; Walsh and Whitehead 1993; Kay 2004; Reed et al. 2007). Crocodilians returning home can cause additional threats to people and their animals, and dealing with these problems can add substantially to management costs. Harvest Killing Targeted Problem Animals: In general this involves a management agency responding to complaints about specific animals and removing the problem animal. This method has been used extensively in the USA to manage problem alligators (Hines and Woodward 1980; Woodward and David 1994; Woodward and Cook 2000) and sometimes in Australia to manage problem Saltwater crocodiles (Letnic 2004; Reed et al. 2004). Targeted problem crocodilian harvest can be done by Government officials, approved private hunters or private individuals/landowners (Hines and Woodward 1980). Some management agencies allow the harvester to sell the skins and meat (Woodward and David 1994; Woodward and Cook 2000). This reduces the costs to the management agency and provides an incentive for authorized hunter/trappers to promptly remove the problem animal (Hines and Woodward 1980). Population Reduction In localized areas where there is a high incidence of human-crocodile encounters, it may be advisable to proactively reduce densities of crocodilians, such as employed by the targeted alligator removal areas in Florida (Woodward and Cook 2000) and the high recreational water use and urban areas in the Northern Territory (Letnic 2004) and Queensland (Kofron 2004). General harvest. Widespread hunting pressure can increase the wariness of crocodilians and reduce the likelihood of human-crocodile conflict. However, this approach may have socio-political acceptance for only those species that are widespread and abundant, such as some populations of American alligators and Nile crocodiles (see Capacity Building - Wild Harvest). References Aust, P., Boyle, B., Ferguson, R. and Coulson, T. (2009). The impact of Nile crocodiles on rural livelihoods in northeastern Namibia. South African Journal of Wildlife Research 39: 57-69. Hines, T.C. and Woodward, A.R. (1980). Nuisance alligator control in Florida. Wildlife Society Bulletin 8: 234-241. Letnic, M. (2004). Crocodile management in the Northern Territory of Australia. Pp. 4-12 in Crocodiles. Proceedings of the 17th Working Meeting of the IUCN-SSC Crocodile Specialist Group. IUCN: Gland, Switzerland. Download. Kay, W.R. (2004). Movements and home ranges of radio-tracked Crocodylus porosus in the Cambridge Gulf region of Western Australia. Wildlife Research 31: 495-508. Kofron, C.P. (2004). The trial intensive management area for crocodiles: a crocodile removal zone in Queensland, Australia. Coastal Management 32: 319-330. Murphy, T.M. and Coker, J.W. (1983). American alligator population studies in South Carolina. Study Completion Report. South Carolina Wildlife and Marine Resources Department: Columbia, SC, USA. Reed, M., Wright, B. and Enoch, C. (2004). Crocodiles in Queeensland - an overview. Pp. 13-27 in Crocodiles. Proceedings of the 17th Working Meeting of the IUCN-SSC Crocodile Specialist Group. IUCN: Gland, Switzerland. Download. Read, M.A., Grigg, G.C., Irwin, S.R., Shanahan, D. and Franklin, C.E. (2007). Satellite tracking reveals long distance coastal travel and homing by translocated estuarine crocodiles, Crocodylus porosus. PLoS One 9: 1-5. Download. Rodda, G.H. (1984). Homeward paths of displaced juvenile alligators as determined by radiotelemetry. Behavioral Ecology and Sociobiology 14: 241-246. Rodda, G. H. (1985). Navigation in juvenile alligators. Zeitschrift fur Tierpsychologie 68: 65-77. Walsh, B. and Whitehead, P. (1993). Problem crocodiles, Crocodylus porosus, at Nhulunbuy, Northern Territory: an assessment of relocation as a management strategy. Wildlife Research 20: 127-135. Woodward, A.R. and Cook, B.L. (2000). Nuisance alligator (Alligator mississippiensis) control in Florida, USA. Pp. 446-455 in Crocodiles. Proceedings of the 15th Working Meeting of the IUCN-SSC Crocodile Specialist Group. IUCN: Gland, Switzerland. Woodward, A.R. and David, D.N. (1994). Alligators (Alligator mississippiensis). Pp. F1-F6 in The Handbook: Prevention and Control of Wildlife Damage. Internet Center for Wildlife Damage Management, University of Nebraska: Lincoln.
4.5.2. Relocation of problem animals
4.5.3. Public Education and Awareness - Colin Stevenson Crocodilian conservation is now a management problem: programs try to manage a growing crocodilian population that lives alongside an often-expanding human population. The inevitable incidents of conflict can generate storms of negative publicity, liability issues, and emotional discussion in the wake of human tragedy. Recent incidents in the USA and Australia highlight this.
(Photos: Yusuke Fukuda) The “No Swimming – Crocodiles” signs have long been a popular souvenir. In producing these signs for sale to tourists, they become a subtle, yet powerful, part of the awareness campaign: tourists assume that countries where such signs are for sale have crocodile populations.
------------------------------------------------------------------------------------------------- |

 Email CSG
Email CSG